Cell Line Development.
Characterization for Cell Line Development and Biologics Production.
In the case of antibodies, early characterization is essential in the initial stages of production. Liquid chromatography mass spectrometry (LC-MS) facilitates sensitive and rapid analysis of antibodies, including:
- Identity: Conducting high-throughput screening and validation of clone pools.
- Purity: Identifying and characterizing protein product variations and contaminants essential for informed cell line engineering.
- Composition: Analyzing post-translational modifications (PTMs), glycosylation patterns, and disulfide bond arrangements to determine favourable production conditions.
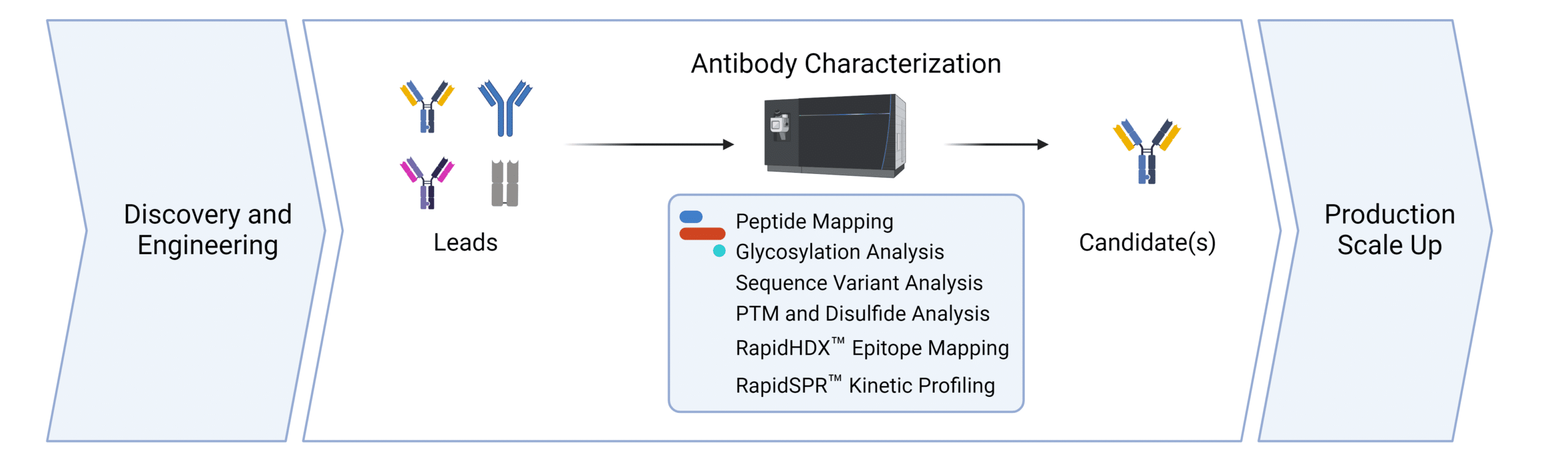
Services for Cell Line Development.
We support the workflows for cell line development at all stages. Contact us to discover how we can help with your cell line development application.
Antibody Sequencing & Discovery Services.
Peptide Mapping
Antibody verification by real-time peptide mapping. Antibody sequence confirmation service for reproducibility.
Explore Peptide Mapping Service
Glycosylation Analysis
Identify the glycan profile and distribution within the host-expression system to facilitate selection and scaling of production.
Explore Glycan Analysis
Sequence Variant Analysis
Identify single point mutations, amino acid substitutions, and contaminants from recombinantly produced mAbs.
Explore Sequence Variant Analysis
PTM and Disulfide Bond Analysis
Discover PTMs and locations of disulfide bonds to optimize biologics production.
Explore PTM Analysis
Explore Disulfide Bond Analysis
SPR Binding Kinetics
Characterize the binding properties of expressed antibody products to target antigens.
Explore SPR Binding Kinetic Service
HDX-MS Binding Kinetics
Characterize linear and conformational epitopes for expressed antibody products against target antigens.
Explore HDX-MS Epitope Mapping Service
Application Publications.
“We’re focused on coupling cytokines with a highly engineered VHVL of an antibody. So by sequencing the antibodies with Rapid Novor we are able to pull out the CDR to graft them into our platform and test in vitro and in vivo.”
Pavel Khrimian – Co-founder and CBO, Deka Biosciences
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics