Vanessa Yoon Calvelo, PhD
Published: April 24, 2023
Introduction
Monoclonal antibodies (mAbs) and related biological products often present as ideal therapeutics largely due to:
- Their high specificity and affinity to the target antigen, allowing for a high level of efficacy with fewer adverse outcomes.
- Their diversity in structure and function, allowing for a wide range of therapeutic applications and targets.
- Their amenability to modifications through antibody engineering and recombinant production, allowing for the generation of many antibody-based derivatives including chimeric antibodies, humanized antibodies, bispecific antibodies, antibody fragments (ie. Fabs, nanobodies, diabodies, triabodies), antibody-drug conjugates, and antibody-PROTACs.
To meet the acceptance criteria of regulatory authorities (ie. FDA, EMA), rigorous programs are required to provide a complete characterization of therapeutic mAbs and related products. This includes:
-
- Structural and physicochemical characterization
- Immunochemical characterization
- Functional characterization
- Purity and contaminant characterization
- Quantification
In this article, we will discuss the different mechanisms of action (MOAs) of therapeutic antibodies and the strategies used to assess their functions.
Functions of Therapeutic Antibodies
MAbs are multifunctional by nature and their efficacy is largely attributed to the antigen-binding fragment (Fab) region and the crystallizable fragment (Fc) region. These two regions can define the antibody’s function and MOA within a specific immune response or biological process. Common MOAs (Figure 1) that drive the biological activities of mAb therapeutics and related products include:
- Blocking of ligand-receptor interactions – mAbs can bind to either side of the ligand-receptor interface to block binding and disrupt the subsequent signal transduction pathway.
- Effector engagement for ADCC, ADCP, and CDC – mAbs can engage effector functions through binding of the Fc region to the Fc receptors and complement proteins to effectively deplete target cells.
- ADCC, or antibody-dependent cellular cytotoxicity, and ADCP, or antibody-dependent cellular phagocytosis, are engaged upon binding of the Fab to the antigen-expressing cell, allowing the mAb to recruit effector cells (ie. natural killer (NK) cells, macrophages) that express Fc surface receptors, which bind to Fc, resulting in the elimination of the mAb-labeled antigen-expressing cell via lysis or phagocytosis.
- CDC, or complement-dependent cytotoxicity, is engaged upon binding of the C1 complex to the antibody-antigen complex, activating a cascade of complement proteins to form an immune complex that is eliminated by effector cells.
- Cytotoxic T cells are engaged through bispecific mAbs to direct the T cells towards tumour cells by binding to both the T cell receptors and the antigen-expressing tumour cell, leading to T cell proliferation and the release of cytotoxic factors (ie. IL-2, INFy).
- Signaling downregulation by receptor internalization and degradation – mAbs binding to nonoverlapping epitopes can be combined synergistically for enhanced internalization and downregulation of epidermal growth factor (EGF) receptors to effectively shut down their signaling pathways that are involved in many human cancer types.
- Targeting by drug delivery – mAbs can be conjugated to cytotoxic payloads (ie. radioisotopes, toxins, small molecules, or cytokines) and delivered directly to tumour cells without causing damage to non-tumour cells.
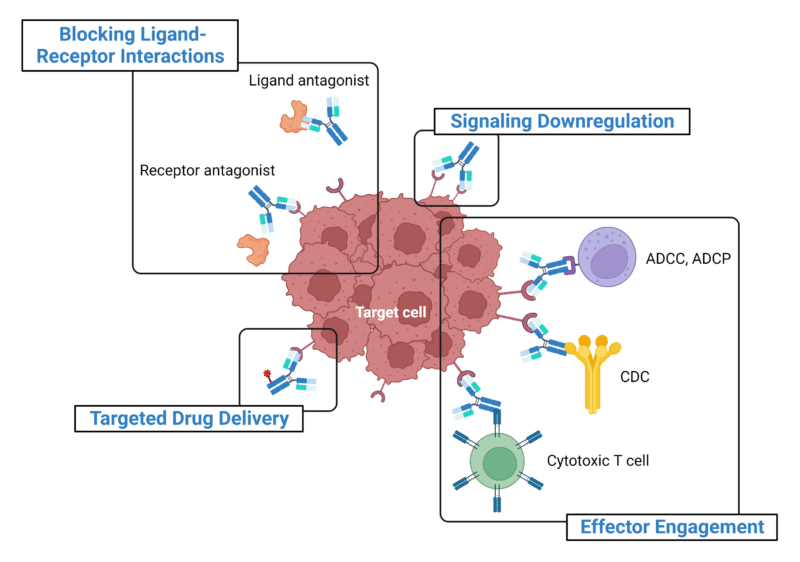
Figure 1. Mechanisms of action of common mAb therapeutics and related products. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, complement-dependent cytotoxicity.
Functional Assays for Therapeutic Antibodies
The biological activity of a mAb is an important property that describes its specific ability or capacity to achieve a biological effect. A comprehensive program encompassing diverse biological assays is often required to measure the biological activity of therapeutic mAbs and related products. This article will focus on the applications of cell culture-based assays for the functional characterization of therapeutic antibodies.
Given that a mAb’s efficacy is attributed to its Fab and Fc regions, the cell culture-based assays can be divided into two categories: Fab-based functional assays and Fc-based functional assays.
Fab-Based Functional Assays
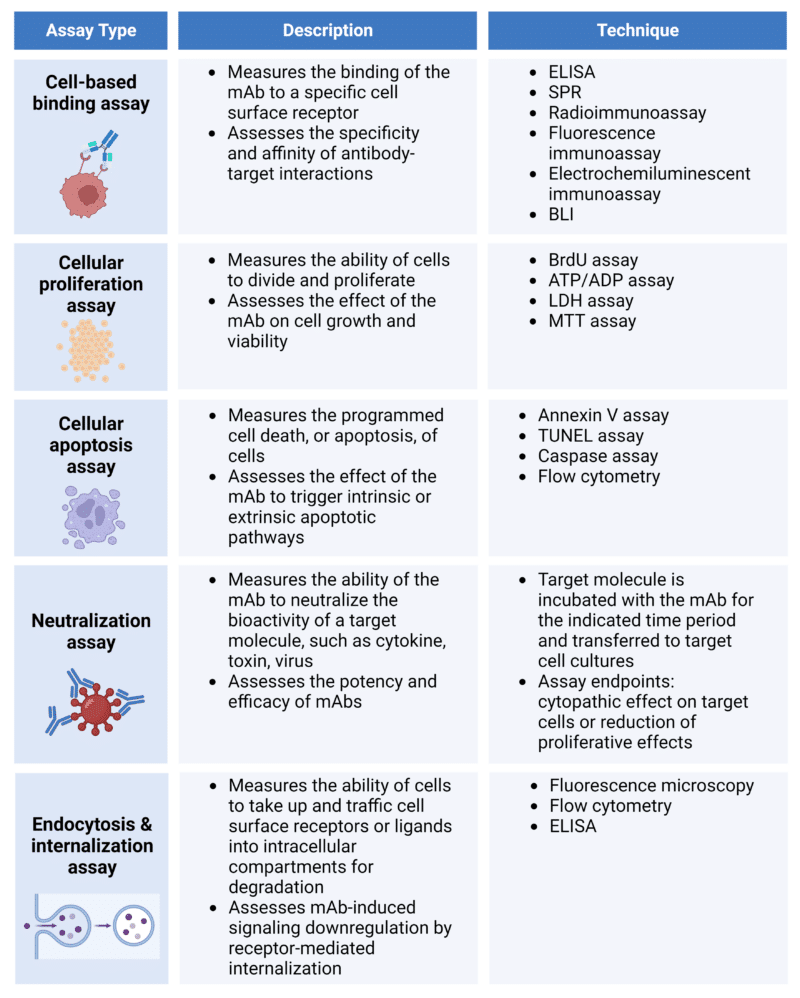
As its name suggests, the Fab region, or antigen-binding fragment, contributes to the binding function of the mAb to its corresponding antigen. Several common functional assays used in assessing Fab-related biological activities are listed in Table 1.
Table 1. Cell-based assays for the characterization of Fab-related biological activities.
Fc-Based Functional Assays
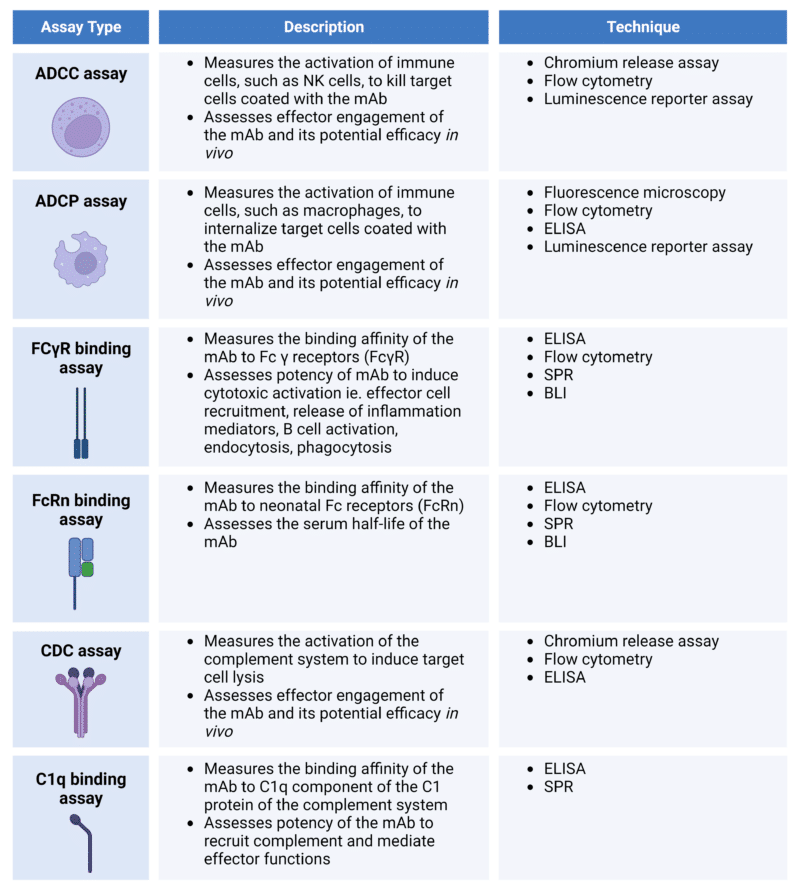
The Fc region ensures that the appropriate immune response is induced upon binding of the Fab region to the given antigen. The binding of the Fc region to specific Fc receptors and complement proteins activates effector functions (see previous section). Several common functional assays used in assessing Fc-related biological activities are listed in Table 2.
Table 2. Cell-based assays for the characterization of Fc-related biological activities.
Functional Characterization in the Therapeutic Antibody Discovery Process
Functional characterization is a key step in the characterization and lead selection stage during the mAb therapeutic discovery process. These assays can provide information on the activity, efficacy, and safety of therapeutic mAbs, which can drive the selection of lead molecules for further engineering and optimization.
It is important to note the distinction between the functional activity and binding activity of an antibody. When referring to the function of an antibody, it is often equated with biological activity rather than binding activity. However, screening for antibody function is challenging for many current antibody discovery platforms. Therefore, these platforms often screen for the initial binding activity to identify potential lead molecules for downstream functional characterization.
The binding of an antibody may not always lead to a biological function. However, binding does need to occur for a functional output, as the antibody cannot carry out its MOA without binding to the antigen. Consequently, assessing binding performance is a crucial step in the therapeutic antibody discovery and development pipeline. Please refer to our article, Immunochemical Characterization of Therapeutic Antibodies, for an overview of the immunochemical properties of therapeutic mAbs, including binding performance, and the diverse analytical techniques used in their characterization.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics