 Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: January 8, 2024
Introduction
Glycosylation plays a crucial role in antibody functions, influencing protein folding, trafficking, stability, and half-life. While the glycosylated residue Asn 297 in the Fc region stands out as the most recognized glycosylation site in antibodies, it is essential not to overlook other glycosylated residues and the importance of the overall glycan profile during antibody and therapeutic development.
Glycoengineering emerges as a promising avenue in the development of next-generation therapeutic biologics, offering opportunities to enhance therapeutic function and safety. Glycoengineering can significantly influence the activity of therapeutic antibodies, affecting pro-inflammatory or anti-inflammatory properties, neutralization capabilities, and the development of antibody-drug conjugates (ADCs).
Exploring glycoengineering in therapeutic antibodies opens new possibilities for tailoring their functions to specific therapeutic needs, ultimately paving the way for improved and more targeted therapeutic interventions.
Glycoengineering of Therapeutic mAbs
Monoclonal antibodies (mAbs) are the largest class of biotherapeutics, and their activity, therapeutic efficacy, and safety is modulated by glycosylation. The extensive diversity of glycan modifications presents several possibilities for glycoengineering, offering a means to modify and customize antibody functions to suit therapeutic applications.
Glycoengineering Pro-Inflammatory and Anti-Cancer mAbs
The pro-inflammatory activity of antibodies include processes like antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement dependent cytotoxicity (CDC). These effector functions are modulated by the fragment crystallizable (Fc) region of IgG antibodies, which bind to Fc receptors on effector cells, such as natural killer cells or macrophages.
N-linked glycosylation in the Fc region containing galactose, core fucose, and sialic acid increases anti-inflammatory activities (Figure 1). The lack of fucose within glycan structures results in subtle conformational changes in the IgG1-Fc region, resulting in increased affinity to the Fc gamma receptor IIIa (Fc𝜸RIIIA). As such, antibodies designed to induce ADCC, ADCP, and CDC, such as anti-cancer therapeutics, are designed to lack these specific sugars.
FDA-approved anti-cancer antibody therapeutics, such as obinutuzumab and mogamulizumab, demonstrate heightened ADCC, ADCP, and CDC activity against cancer cells due to the absence of fucosylation, or afucosylation. Glycoengineering efforts involving the removal of terminal sialylation in pertuzumab have also proven successful in enhancing ADCC and CDC activity for treating HER2-positive breast cancer.
Human derived antibodies exhibit high fucosylation, with afucosylated antibodies constituting a range of 1.3-19.3% in the native antibody repertoire. Similarly, antibodies produced through recombinant methods in Chinese hamster ovarian (CHO) cells display a high fucosylation rate, up to 90%. CHO cells also exhibit a low sialylation rate, where ~98% of recombinantly produced antibodies are asialylated. The high fucosylated glycan profiles observed in both human-derived and CHO-produced antibodies has resulted in glycoengineering efforts to modify glycosylation profiles. These efforts aim to produce afucosylated and asialylated antibodies, enhancing their proinflammatory applications, particularly in the context of anti-cancer therapeutics.
Glycoengineering Anti-Inflammatory mAbs for Autoimmune Diseases
Anti-inflammatory antibodies exert their effects through diverse mechanisms, targeting specific components of the immune system to mitigate inflammation. These mechanisms include cytokine neutralization, inhibition of immune cell activation, complement inhibition, and blocking immune cell migration. Several anti-inflammatory antibodies are used to treat conditions such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and psoriasis.
In contrast to the absence of fucose and sialic acid, the presence of these glycans contributes to anti-inflammatory functions in antibodies used to treat autoimmune diseases by impairing ADCC and CDC (Figure 1).
Intravenous immunoglobulin (IVIG) serves as an IgG replacement therapy for immunocompromised individuals and patients with inflammatory or autoimmune diseases. Derived from pooled IgG collected from the plasma of numerous healthy donors, approximately 10-15% of serum IgG in IVIG is sialylated. Notably, sialylated IgG, particularly α-2,6 sialylation, plays a crucial role in the anti-inflammatory activity associated with IVIG treatment.
The nature of CHO cell production systems allows for the development of anti-inflammatory antibodies due to high fucosylation, and residual sialylation levels. However, efforts to increase the sialylation of therapeutic antibodies may enhance the effectiveness of these drugs.
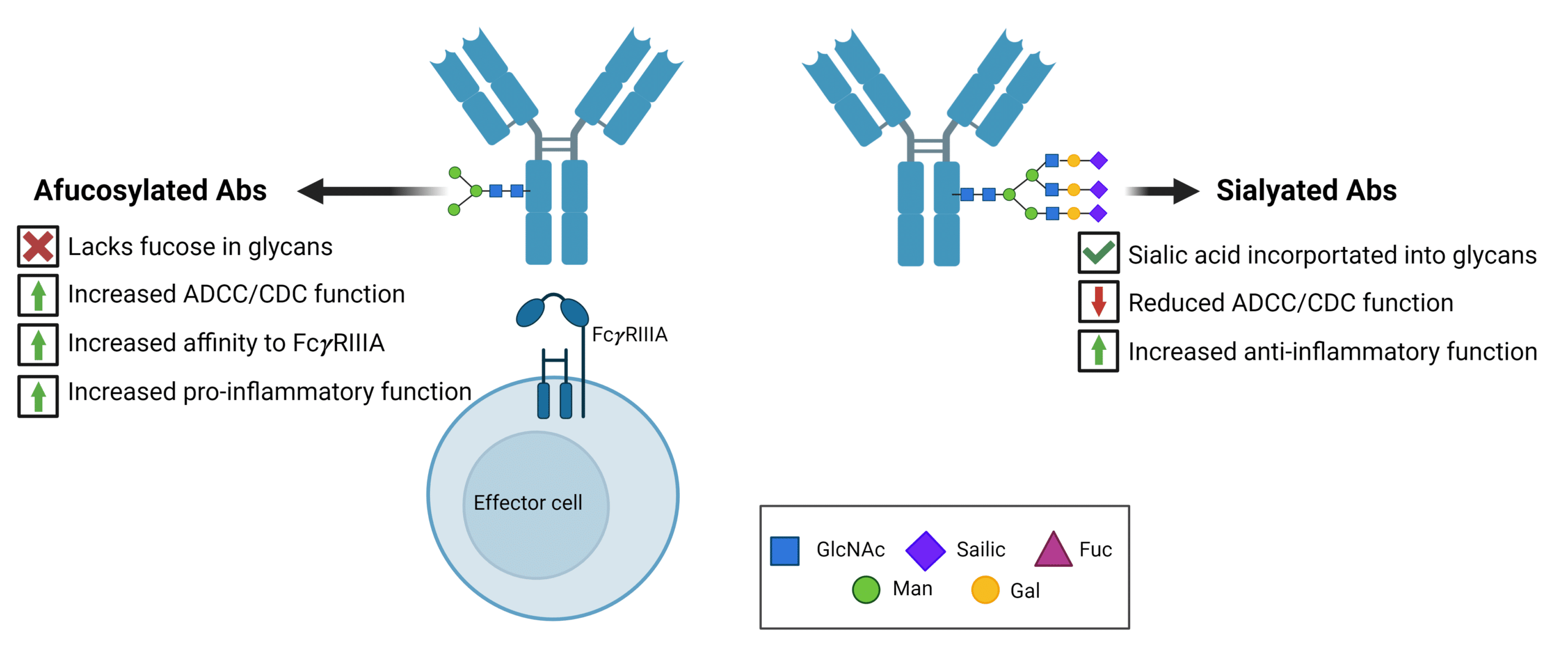
Figure 1. Overview of pro-inflammatory and anti-inflammatory antibody functions mediated by glycan modifications. Afucosylated antibodies increase inflammation through increased ADCC/CDC function via increased affinity to the Fc𝜸RIIIA receptor. Sialylated antibodies reduce ADCC/CDC function, promoting anti-inflammatory cellular activity.
Glycoengineering to Improve Antibody Neutralization
Neutralizing antibodies diminish or eliminate viral infections by blocking viral entry and replication. Antibodies that exhibit potent neutralizing activity are often considered essential for effective immunity against specific viruses, making them valuable candidates for therapeutic intervention against viral infections.
Ibalizumab serves as an example, representing a humanized monoclonal antibody that blocks HIV-1 entry by binding with high affinity to human CD4 receptors. HIV-1 strains resistant to ibalizumab exhibit a mutation resulting in the loss of an N-linked glycan in the V5 loop of HIV-1. Structural analysis has revealed that this N-glycan occupies the space between the V5 loop and the light chain of ibalizumab. By engineering ibalizumab with N-linked glycosylation on the light chain, its ability to neutralize previously resistant strains of HIV-1 is restored.
Engineering Glycosite-Specific ADCs
Antibody-drug conjugates (ADCs) represent a promising class of anti-cancer therapeutics designed to precisely deliver chemotherapeutic payload drugs to target tissues, minimizing off-target effects. The drug payload of ADCs is covalently attached to the antibody, often through amine groups of lysine or thiols of cysteine residues dispersed across the amino acid sequence of the antibody. A major downfall of this approach is that it results in a heterogeneous pool of ADCs, characterized by different drug to antibody ratios (DAR), posing challenges to clinical efficacy, safety, and cytotoxicity.
Glycoengineering presents an avenue to create homogenous ADC pools by creating glycosite-specific ADCs (gsADCs). By leveraging the conserved N-glycosylation site N297 on the Fc domain, the engineering of a thiol-modified fucose allows for site-specific payload conjugation, providing a uniform and controlled drug distribution on the antibody. This innovative approach holds promise for addressing the limitations associated with ADC heterogeneity, potentially enhancing their clinical performance and therapeutic impact.
Glycoengineering Methods
Various methods exist for engineering glycan modifications on antibodies, each with its advantages and limitations. The choice of method depends on the specific goals of the glycan modification, the nature of the glycoprotein of interest, and the desired level of control over the modification process. Generally, glycoengineering methods can be divided into two approaches: cell-based and cell-free.
Cell-Based Glycan Modification Methods
Cell-based glycan engineering utilizes common expression hosts for recombinant protein production and can be performed in yeast, plant, insect, and mammalian cells. The glycosylation machinery and capabilities differ significantly between species, affecting both the sites of glycan attachment and the attached glycans. Approximately 70% of recombinant proteins are expressed in CHO cell lines making them predominant in glycan engineering efforts, particularly for antibodies.
Genetic methods involve manipulating glycotransferase enzymes, and/or other genes involved in glycan biosynthesis pathways. Gene overexpression, or knockdown with RNA interference (RNAi) or knockout strategies with genome editing can alter glycosylation patterns genetically. For example, a FUT8 knockout cell-line can generate afucosylated antibodies by disrupting the α-1,6 fucosyltransferase enzyme encoded by the FUT8 gene (Figure 2).
However, due to the complexity of endogenous glycosylation processes it is challenging to gain precise control and homogenous glycan modifications. Additionally, achieving high-efficiency knockdowns may result in inconsistent and undesirable glycosylation modifications.
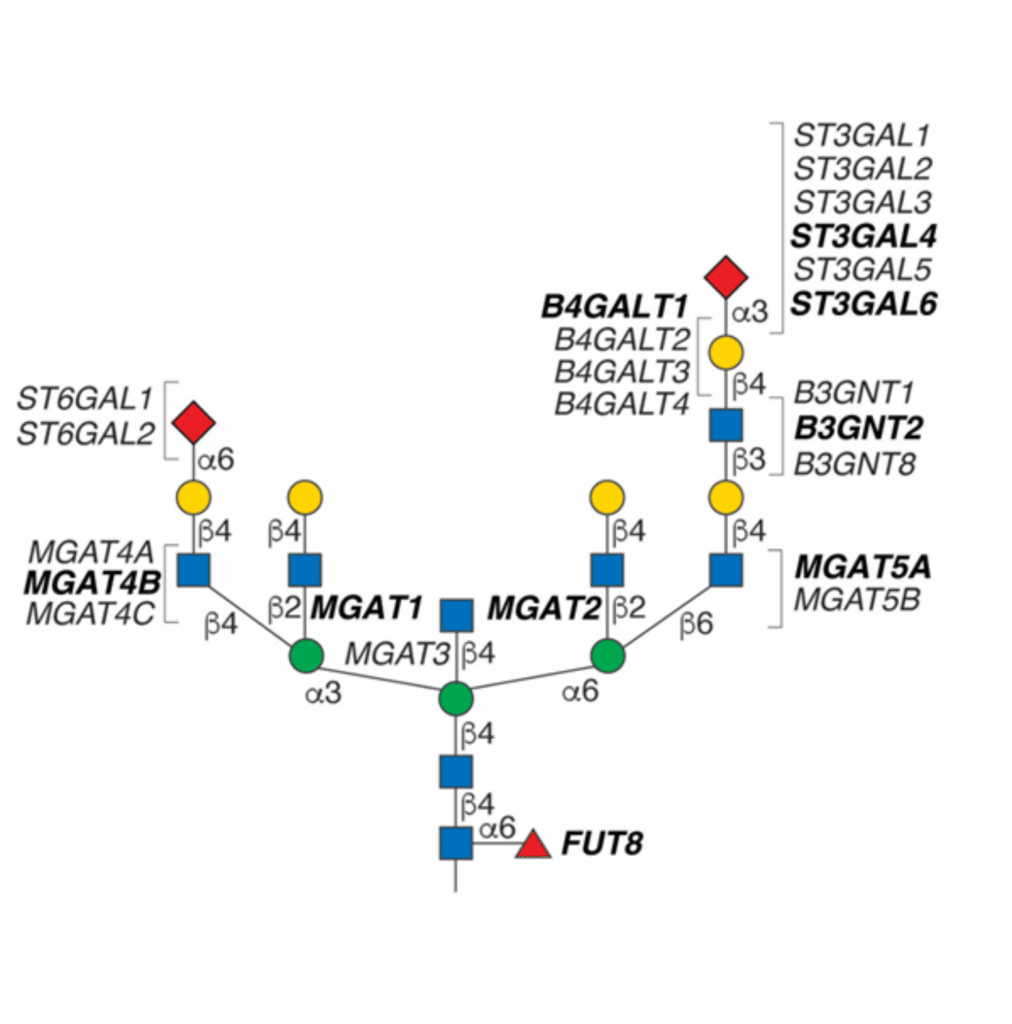
Figure 2. Different glycotransferase enzymes are responsible for each N-glycan branching, elongation, and sialylation reaction in CHO cells. Knockouts of various glycotransferase enzyme genes can disrupt glycosylation pathways, leading to alterations in glycan structures. Figure adapted from Clausen et al., 2022.
Metabolic engineering is a common method used for glycan modifications with non-naturally occurring sugars. Non-natural sugar analogs are incorporated into glycan structures by glycotransferases, but their competition with natural sugars may lead to inconsistent and sub-stoichiometric incorporation into glycoproteins.
Cell-Free Glycan Modification Methods
Cell-free glycan modification strategies offer the potential for more specific control over glycosylation patterns, resulting in homogenous and structurally defined glycoproteins.
Chemical synthesis and derivation methods are used to synthetically synthesize glycan components, followed by their conjugation through a series of steps involving stepwise chemical reactions, solid-phase synthesis, and native chemical ligation. While significant progress has been made in total synthetic approaches, large-scale therapeutic manufacturing remains a limitation.
Chemoenzymatic methods for glycan engineering is an emerging tool that can develop homogenous glycoproteins in high yields. This method involves the trimming of native heterogenous glycans, followed by the re-glycosylation through activated donor glycans by a glycosynthase (Figure 3). Obtaining pure glycans in sufficient quantities and employing efficient chemical activation methods are crucial considerations in this approach.
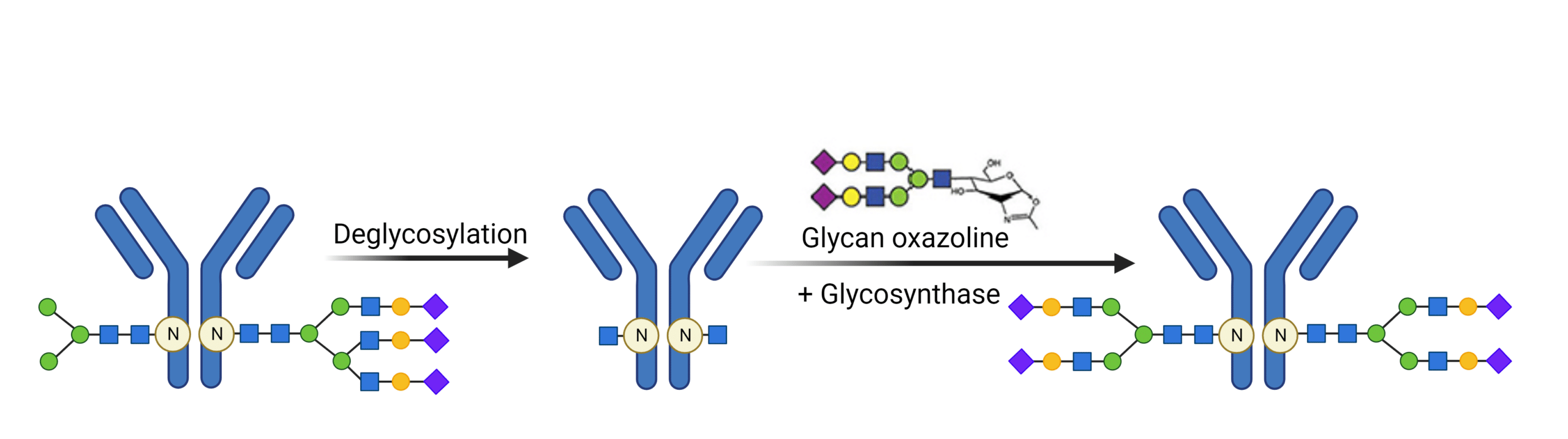
Figure 3. Chemoenzymatic method for glycan engineering homogenous glycan forms.
Additional cell-free antibody engineering techniques may be employed to manipulate glycans, including modifications to amino acid sequences for the removal of glycosylated residues or Fc-swapping to incorporate Fc domains with desired glycan modifications and effector functions.
N-Glycan Analysis Service with Rapid Novor
Antibody glycosylation extends beyond the Fc glycan, encompassing a diverse range of glycan profiles and sites that can modulate antibody functions for specific therapeutic purposes. At Rapid Novor, we utilize liquid chromatography and mass spectrometry (LC-MS) to identify the locations and relative quantities of common glycans, providing comprehensive insights into antibody glycosylation.
For more information on glycan analysis, contact our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

