Nichakawade, T.D., Ge, J., Mog, B.J. et al. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers. Nature 628, 416–423 (2024). https://doi.org/10.1038/s41586-024-07233-2
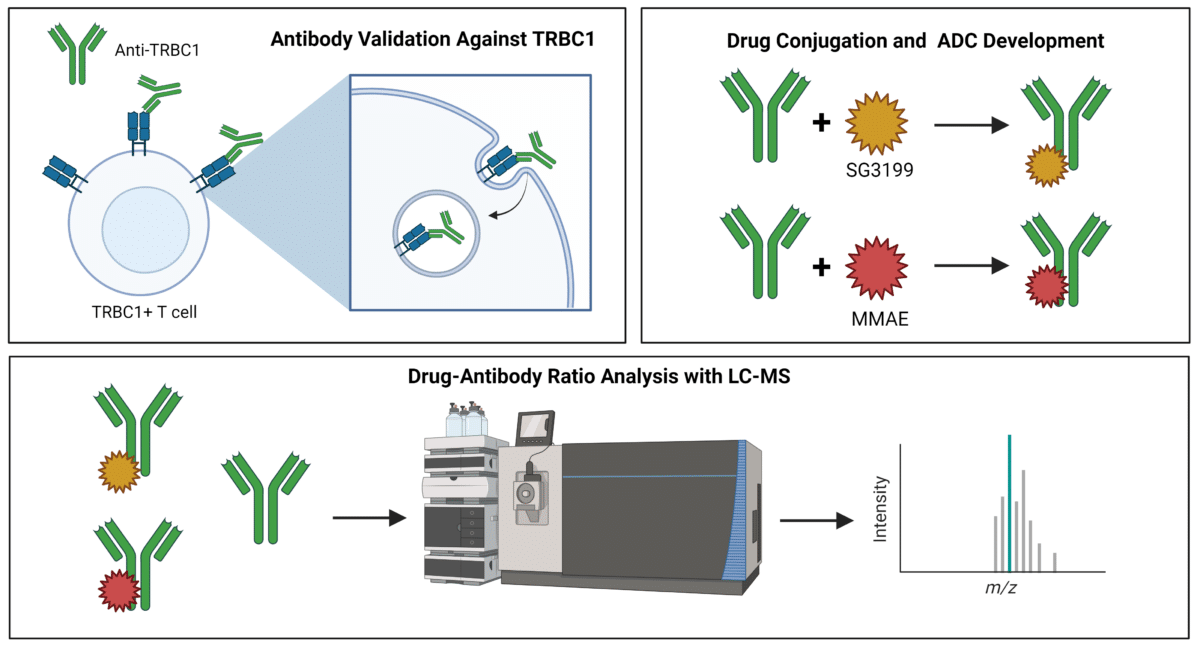
Using antibodies that target TRBC1 on the surface of T cells and become internalized, researchers at Johns Hopkins developed antibody-drug conjugates (ADCs) to target cancerous TRBC1+ T cells. They employed Drug-to-Antibody Ratio (DAR) analysis, crucial for ADC development, to ensure optimal drug delivery to cancer cells while minimizing off-target effects and toxicity.
Key Takeaways
- T cell leukemias and lymphomas arise from clonal expansions with identical TCR sequences in cancerous T cells, differing from normal T cells in TRBC1 or TRBC2 expression.
- Anti-TRBC1 CAR-T cell therapy, though attempted, failed in clinical trials due to poor expansion.
- Researchers at Johns Hopkins developed an anti-TRBC1 ADC, selecting SG3199 and MMAE as conjugated cytotoxic drugs, achieving DARs of 2.4-2.6 and 3.2 respectively.
- LC-MS-based characterization ensured precise DAR determination critical for efficacy in targeting and killing TRBC1+ cancer cells while sparing normal cells, demonstrating promising results in preclinical models.
Summary
T cell leukemias and lymphomas originate from the clonal expansion of a specific T cell, resulting in every cancerous T cell having the same unique TCR sequence. Normal T cells have an equal distribution of cells expressing TRBC1 and TRBC2, whereas T cell cancers express either TRBC1 or TRBC2, but not both. CAR-T therapies targeting TRBC1 looked promising, but failed in clinical trials.
In 2023, researchers from the Dr. Suman Paul lab at The Johns Hopkins University School of Medicine aimed to develop an antibody therapeutic to target TRBC1 + cells that is not dependent on CAR-T cells.
Antibody-drug conjugates kill target cancer cells by recognizing cancer-specific antigens on the cell surface, leading to the internalization and release of cytotoxic drugs. They tested and selected an anti-TRBC1 antibody that bound to TRBC1+ TCRs on T cells and internalized. Following this, they tested antineoplastic drugs and chose the top two performing drugs, SG3199 and monomethyl auristatin E (MMAE), to conjugate to their anti-TRBC1 antibody.
Following the synthesis of the ADCs, LC-MS-based antibody characterization by Rapid Novor was used to determine the drug-antibody ratio (DAR). The DAR was estimated at 2.4 to 2.6 for anti-TRBC1–SG3249 and 3.2 for anti-TRBC1–MMAE, based on the peak area analysis from the mass spectra.
In vitro and in vivo experiments were conducted to evaluate the effectiveness of the ADC. The ADC demonstrated a greater potency in killing TRBC1+ cancer cells compared to TRBC1+ normal T cells. In vivo efficacy was assessed using TRBC1+ T cell cancer xenografts. Following treatment with anti-TRBC1–SG3249, the cancers were undetectable, and no recurrences were observed.
Understanding the specific TCR sequences in cancer led researchers to explore ADCs as an alternative to CAR-T cell therapies, which faced limitations in clinical trials. Accurate DAR analysis, crucial for ADC development, ensures optimal drug delivery to cancer cells while minimizing off-target effects and toxicity. This analytical approach is essential for regulatory approval and maintaining manufacturing consistency.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

