Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: September 17, 2024
Introduction
In the last decade, immunotherapy has rapidly transformed treatments for cancer, autoimmune disorders, and infectious diseases. While the market is dominated by antagonistic antibodies that inhibit biological pathways, there is growing interest in agonistic antibodies, which activate these pathways. These antibodies uniquely engage and modulate cell surface receptors, acting like natural ligands to trigger specific cellular responses. This targeted signaling makes them a promising therapeutic tool for complex diseases. However, despite their potential, most approved antibody therapies are still antagonists. This discussion will explore the challenges of discovering and developing agonistic antibodies.
Agonistic Antibody Mechanism of Action
Unlike antagonistic antibodies that primarily function by blocking or neutralizing their targets, agonistic antibodies work by activating specific biological pathways, effectively mimicking the action of natural signaling molecules (Figure 1). Agonistic antibodies can restore or enhance biological functions that have been compromised by disease. They mimic natural ligands, binding to receptors allosterically, promoting receptor dimerization or clustering, stabilizing ligand-receptor interactions, or recruiting additional co-factors essential for receptor activation.
- Signal Pathway Activation: Agonistic antibodies can activate various signaling pathways in various different manners. For example, anti-CD40 antibodies simulate the action of the CD40 ligand, enhancing B cell activation and overall immune responses. Similarly, XMetA antibodies bind allosterically to the insulin receptor, improving glucose regulation in diabetic patients. Anti-DR5 antibodies induce receptor multimerization, leading to cancer cell apoptosis. Nanobodies targeting IL-6 stabilize receptor-ligand interactions, boosting signal transduction. Anti-CD137 antibodies facilitate the recruitment of TNFR-Associated Factors (TRAFs), essential for downstream immune activation.
- Immune System Activation: Agonistic antibodies can significantly enhance the immune response by either directly stimulating immune cells or by modulating immune checkpoint molecules. For example, Ipilimumab, an agonistic antibody targeting CTLA-4, reduces the inhibitory effects on T cells, thereby boosting their ability to attack tumors. Urelumab targets CD137, a co-stimulatory molecule on T cells and natural killer cells, enhancing their proliferation and activation to strengthen the immune response against cancer. αGITR (agonistic anti-GITR antibody) binds to the glucocorticoid-induced TNFR-related receptor (GITR) on regulatory T cells (Tregs), diminishing their suppressive function and enhancing the activation of effector T cells for improved anti-tumor immunity.
- Cell Death or Proliferation Activation: Some agonistic antibodies induce cell death through mechanisms such as apoptosis, necroptosis, antibody-dependent cellular cytotoxicity (ADCC), or complement-dependent cytotoxicity (CDC). For instance, the agonistic antibody TR2-3 has been shown to induce apoptosis in cancer cells by upregulating the cleavage of caspase-3 and caspase-8, key proteins in the apoptotic pathway. Conversely, in conditions where cell survival is crucial, such as ischemic diseases, agonistic antibodies can promote cell proliferation. An example is the agonistic antibody Ab4B19, which activates TrkB, leading to the rescue of neurons from necroptosis during ischemic injury.
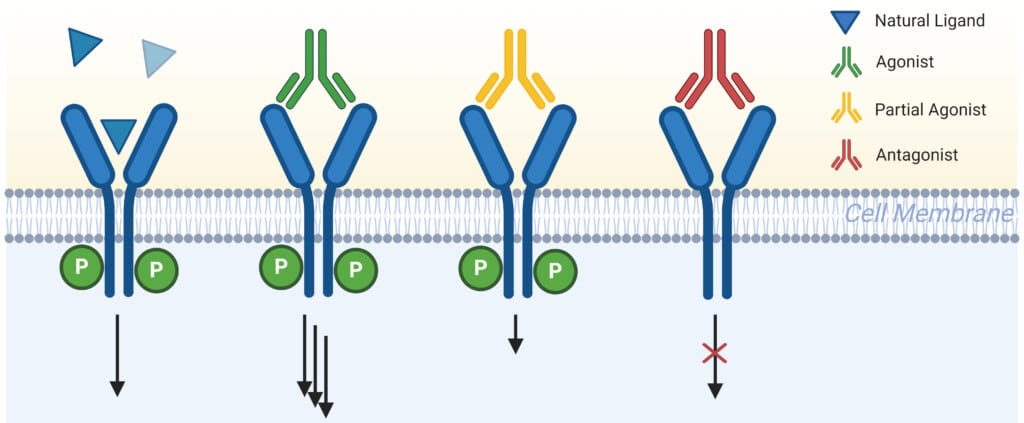
Figure 1: Schematic Outlining the Differences in Receptor Modulation Between Agonist, Partial Agonist, Antagonist, and Natural Ligand.
Agonistic Antibody Development Challenges
Since the commercialization of the first therapeutic monoclonal antibody in 1986, only one agonist antibody—sotigalimab—has received FDA approval. In fact, there are very few agonistic antibodies in advanced clinical trials. Why is the development of agonistic antibody therapeutics lagging behind that of antagonistic antibodies? Let’s delve into the complexities involved in receptor agonism.
A major challenge with agonistic antibodies is their intricate mechanism of action. Unlike antagonists, which simply block receptors, agonists must induce specific conformational changes to activate receptors, which can be difficult to control. For instance, CD28-SuperMAB (TGN1412) was designed to activate T cells but instead caused a dangerous cytokine storm due to difficulties in precisely controlling receptor activation. Similarly, anti-CD40 antibodies, which require careful cross-linking for effectiveness, have shown inconsistent therapeutic outcomes due to challenges in achieving reliable receptor engagement. In fact, conventional antibodies are not able to mediate the higher-order receptor clustering that is needed to activate receptors that are clinically relevant. These examples highlight that binding assays alone are insufficient for confirming agonistic function; instead, complex functional assays are necessary.
Pharmacokinetic and pharmacodynamic issues add further complexity to the development of agonistic antibodies. These antibodies can lead to receptor internalization and degradation, which shortens their half-life and complicates dosing schedules. For example, anti-DR5 antibodies have faced such issues, resulting in less predictable therapeutic profiles compared to antagonist antibodies. Additionally, the dynamic nature of receptor signaling, as observed with agonistic anti-CD137 antibodies, leads to non-linear dose-response relationships, complicating their clinical application.
Most importantly, the underlying biology of receptors targeted by agonistic antibodies is not fully understood, contributing to their development challenges. Variability in success among anti-CD137 antibodies reflects the ongoing efforts to elucidate the precise signaling pathways required for effective immune activation. Clinical trials have revealed unexpected liver toxicity, likely due to the activation of 4-1BB on non-target cells. This highlights the need for a thorough understanding of target receptor expression in non-cancerous tissues and potential off-target effects before therapeutic application. Moreover, many receptors have additional, less well-understood activities. For example, death receptor activation is involved not only in apoptosis but also in cell proliferation, survival, and angiogenesis.
Optimizing Agonistic Antibodies
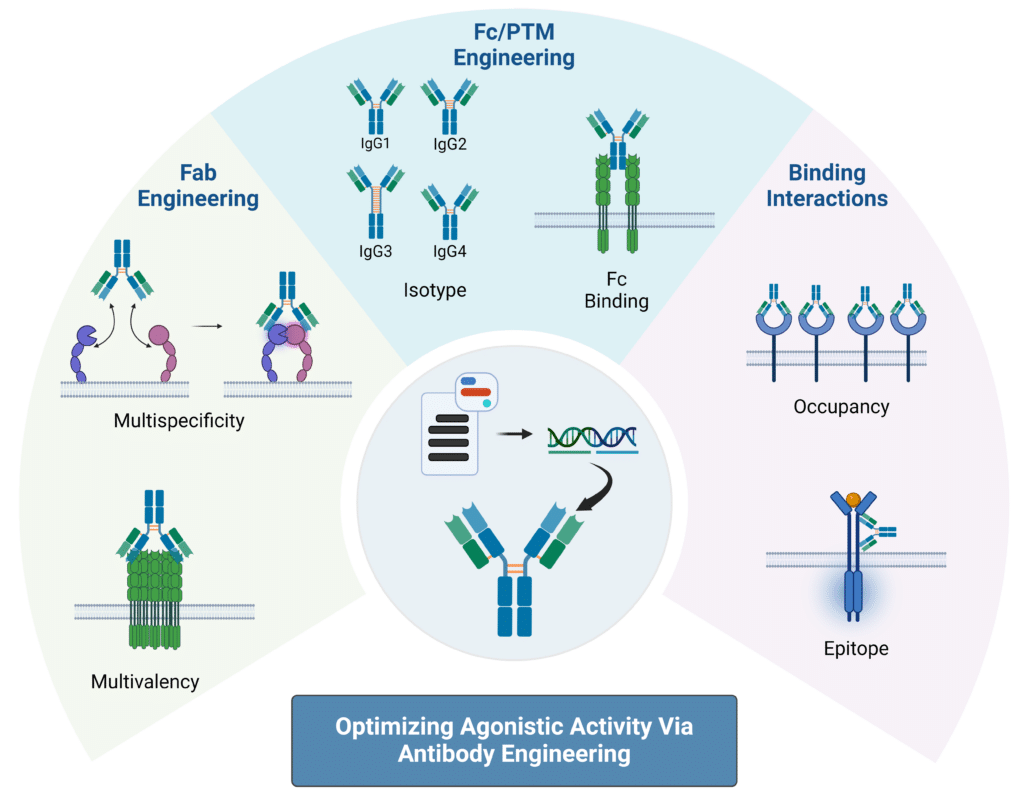
In order to develop agonistic antibodies that are safe and potent immunotherapies, optimization of current methods are necessary. Key properties to optimize for agonistic antibody development are valency, isotype selection, Fc region and receptor interactions, and epitope location. The details of using these properties to “fine-tune” receptor agonist antibodies to increase therapeutic potential are as follows (Figure 2):
Valency
The number of antigen-binding sites on an antibody, or valency, can enhance the therapeutic potential of receptor agonist antibodies. By increasing valency, these antibodies can achieve more effective receptor cross-linking and activation, leading to a stronger sustained signal. Additionally, higher valency can help overcome resistance mechanisms by engaging multiple receptors or pathways simultaneously. To do this, engineering can be used to design multivalent constructs such as tetravalent or bispecific antibodies. For instance, one study increased GITR activation by 2.5 fold by engineering a novel hexameric Fc-fusion protein. Another study engineered nanobodies that were fused together to create multivalent constructs that activated DR5 to the level of the natural ligand.
Multispecific
Multispecific antibodies, which target both tumor-associated antigens and T cell receptors, are emerging as promising tools for enhancing the efficacy of agonist antibodies. These antibodies can minimize off-target effects by directing therapeutics specifically to the tumor microenvironment. Recent studies have demonstrated that bispecific antibodies targeting both DR5 and fibroblast-activation protein (FAP) or CD137 and OX40 show significant improvements in antitumor activity and drug localization. Other multispecific antibodies have also shown enhanced T cell proliferation and cytokine production, highlighting their potential in boosting antitumor immunity. Many clinical trials are either underway or completed to evaluate these bispecific antibodies, including RG7827, ATOR-1015, and RO7300490, all pointing towards a growing interest in their development for cancer therapies.
Isotype
Antibody isotype selection plays a role in the therapeutic potential of receptor agonist antibodies. Different antibody isotypes have distinct properties and effector functions. For example, IgG1 and IgG3 are effective in mediating antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) due to their strong Fc receptor interactions, while IgG4 is less likely to engage these pathways, reducing potential off-target effects. Another major property to consider is the differences in disulfide bonds between the different IgG isotypes. These bonds play a crucial role in stabilizing the antibody structure; differences in isotype-specific disulfide bonding patterns can affect the antibody’s hinge region flexibility, stability, folding, and functional efficacy. In humans, IgG2 has shown superior agonistic activity for several targets, including CD40, 4-1BB, and CD27, partly due to its unique hinge structure that promotes a compact conformation beneficial for receptor clustering. By selecting the appropriate isotype and engineering disulfide bonds, you can optimize the stability and therapeutic effectiveness of receptor agonist antibodies.
Fc Region Receptor Interaction
Engineering antibody Fc regions to enhance interactions with Fcγ receptors can significantly boost agonist activity. One approach involves introducing mutations that increase affinity for FcγRIIB, which acts as a scaffold to cluster monomeric receptors and improve agonist effects. For instance, one study introduced mutations in anti-CD40 antibodies that lead to selective engagement with FcγRIIB over FcγRIIA which caused a significant increase in T cell activation leading to a dramatic reduction in tumor growth. Additionally, Fc mutations that promote self-interactions or hexamerization, such as E345R, can enhance receptor clustering and agonist activity independently of FcγR availability. These strategies have been validated in preclinical and clinical trials, showing promising results in enhancing anti-tumor responses and advancing the development of Fc-engineered antibodies for cancer immunotherapy. Strategies to enhance agonist efficacy include Fc engineering to improve interaction with FcγR, using alternative receptors for cross-linking, or employing FcγR-independent approaches like hIgG2 to simplify administration.
Epitope Location
Targeting specific domains of TNF receptors, such as the cysteine-rich domains (CRDs) outside the ligand-binding region, can enhance agonist antibody activity. Antibodies that bind to distal CRD1 domains show greater agonist function compared to those targeting proximal domains, such as CRD3 and CRD4. This is due to the fact that targeting distal domains can reduce steric hinderance and allow for better access to Fcγ receptors. For example, urelumab, which binds to CRD1, induces significantly more receptor clustering and cytokine secretion than the ligand-blocking antibody utomilumab. Additionally, studies highlight that optimal agonist activity is achieved at intermediate receptor occupancy levels, with antibodies like BMS-986178 showing peak function at around 40% receptor occupancy. This phenomenon is crucial for designing effective cancer therapies, as seen in ongoing clinical trials for various TNF receptors.
Figure 2: Strategies for Optimizing Agonistic Activity Using Antibody Engineering Techniques.
Agonistic Antibody Development
For effective development of agonistic antibodies, Rapid Novor offers a range of services tailored to streamline and enhance your research.
- Antibody Discovery with REpAb: Sequence functional antibodies directly from the immunoserum, ensuring that the starting material already contains functional antibodies that may exhibit agonistic functionalities. Protein purification and enrichment strategies can better isolate rare and functional clones to regions of interest.
- Kinetic analysis with SPR: Measures real-time binding kinetics and affinity of agonistic antibodies to their targets.
- HDX-MS: Identify binding sites of agonistic antibodies on target receptors, as well as conformational shifts in receptors triggered by antibody binding.
By integrating these services, you can effectively advance your agonistic antibody projects, addressing key challenges and enhancing the development of potent therapeutics. For more details, contact our scientists today!
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics