Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: December 20, 2024
What is Phage Display?
Over thirty-five years ago, phage display technology was developed by the pioneering work of John McCafferty and Sir Gregory Winter, which allowed peptides to be displayed on viral surfaces for binding affinity screening. After refinement of this work by Smith and others, full antibody libraries can now be displayed on bacteriophages dramatically altering the way we discover therapeutic antibodies.
Phage display technology has since played a critical role in developing monoclonal antibodies for treating cancer, autoimmune disorders, and infectious diseases. Up to now, numerous clinical trials have been conducted to examine phage-derived antibodies therapeutic potential, with well over a dozen being approved for therapeutic use in humans. However, phage display comes with inherent challenges that require the development of innovative tools to address them.
How Does Phage Display Work?
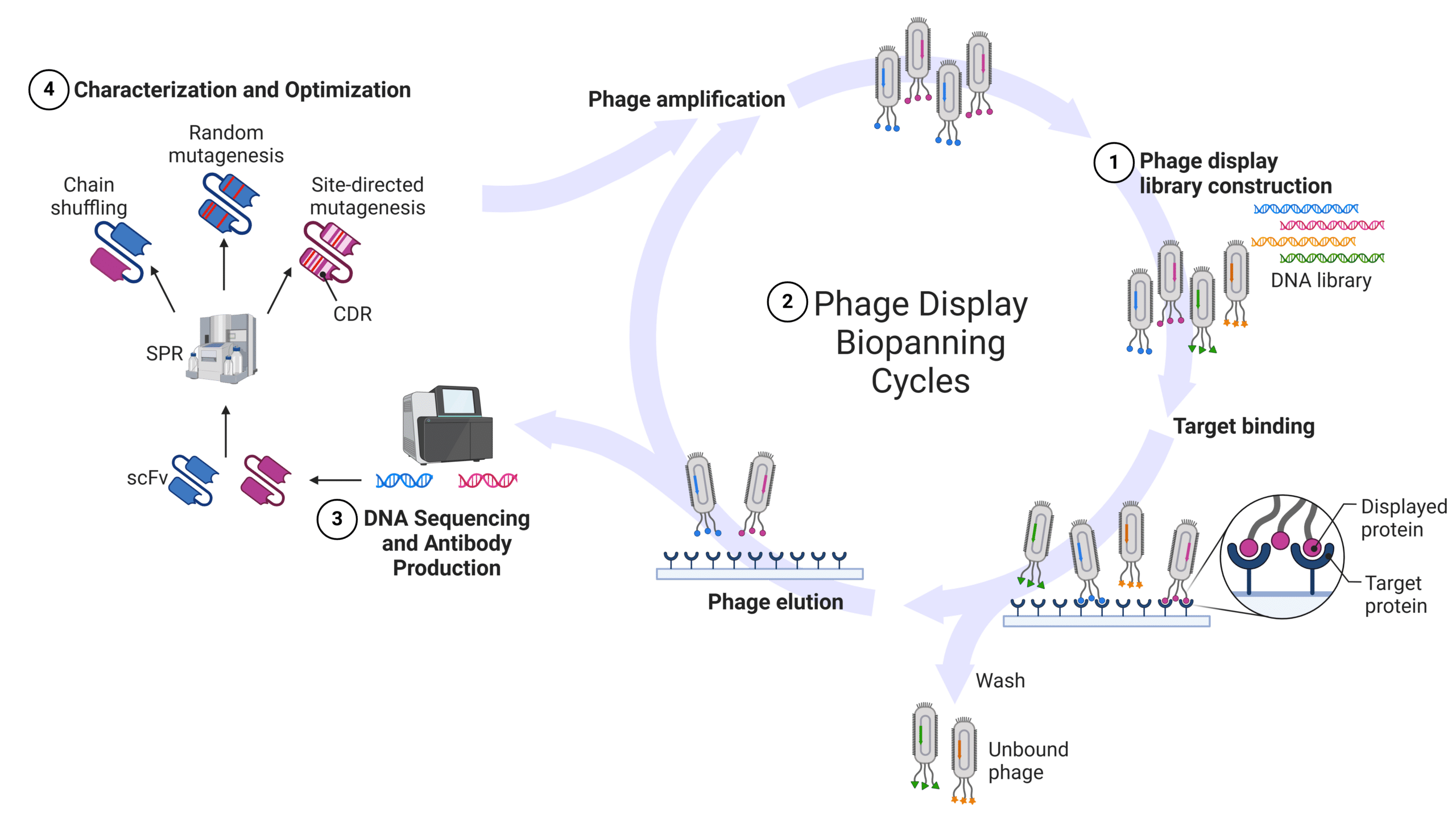
Phage display is a laboratory technique used to study interactions between proteins, such as antibodies, by displaying them on the surface of bacteriophages. Researchers can then use this phage display library to screen the antibodies to identify the ones that bind specifically to a target, such as a receptor, antigen, or other biomolecule. This allows for the identification of high-affinity antibodies that can be used in diagnostics, drug targeting, epitope mapping, and protein interaction studies. The process for antibody discovery is as follows (Figure 1):
- Library Construction: Constructing a library for phage display technology begins with creating a diverse genetic library from either immune or non-immune sources. For example, immune libraries can be sourced from B cell repertoire DNA, while naive libraries can be created synthetically to generate random antibody sequences. Once the genomic library has been synthesized it is inserted in the bacteriophage genome such that when the antibodies are expressed, they are fused to the coat protein of the bacteriophage. Filamentous M13 bacteriophage is typically used for antibody display and the antibody gene is usually inserted into the pIII or pVIII gene. The genetic material can encode antibody fragments, such as antigen binding fragment (Fab), single chain variable fragment (scFv), and nanobodies, or the full antibody sequence. This process generates a highly diverse antibody library that can be further explored for binding affinity to the antigen of interest.
- Phage Display Biopanning Cycles: Biopanning, or panning, is the process of isolating bacteriophages that express antibodies specific to the antigen of interest. This targeted selection process begins with exposing the bacteriophages display library to a surface-immobilized antigen of interest. The phage with antibodies specific to the antigen of interest will bind respective epitopes. This is followed by stringent washing to remove weakly bound or non-specific bacteriophages. After the wash step, the bacteriophage that are bound to the antigen of interest are eluted using enzymatic cleavage or pH change and used to infect E. coli cells for amplification of the antigen-specific bacteriophage. The whole biopanning cycle is typically repeated three to five times in order to enrich the bacteriophage with the highest affinity and specificity.
- DNA Sequencing and Antibody Production: After biopanning is complete, individual phage clones from the selected pool are isolated, and the genetic sequences of the antibodies are obtained through DNA sequencing. This data is essential for the next step, which involves producing the identified antibodies. Once the genetic sequence is determined, the corresponding antibodies can be expressed and generated in different forms, such as scFv or full-length antibodies, for further evaluation and analysis.
- Characterization and Optimization: The produced antibodies then undergo characterization assays to evaluate specificity, affinity, and function. This can involve assays such as an enzyme-linked immunosorbent assay (ELISA) or surface plasmon resonance (SPR) assay. Depending on the desired level of specificity and affinity, the antibodies can then be subject to rigorous optimization assays. This usually involves many different antibody diversification methods followed by rounds of phage display biopanning cycles to achieve in vitro affinity maturation. Antibody diversification methods include chain shuffling, random mutagenesis, and site-directed mutagenesis. These diversification methods have been successfully used to improve the affinity of antibodies like moxetumomab pasudotox and ranibizumab.
Figure 1: Overview of Phage Display Technology workflow.
Phage Display Technology Advantages
The use of phage display technology provides several key advantages that make it particularly effective for generating therapeutic antibodies. One of its key strengths is high-throughput screening, allowing researchers to rapidly identify target antigen binders in a single experiment. The antibody libraries can consist of billions of unique clones, each displaying a different antibody or antibody fragments. This diversity facilitates high-throughput screening, which significantly accelerates the discovery process compared to traditional hybridoma-based methods.
The versatility of phage display is another major benefit, enabling the identification of antibodies or peptides that bind to a wide range of targets, including small molecules, proteins, nucleic acids, and even complex structures like whole cells. It is also cost-effective compared to traditional methods like hybridoma technology, particularly for generating large numbers of diverse binders, while requiring only small sample volumes, reducing reagent costs. Additionally, unlike other antibody generation methods, phage display is an in vitro process, eliminating the need for animal immunization. This reduces ethical concerns and costs associated with animal use.
Another advantage is the precise control over antibody composition. Researchers can generate fully synthetic antibodies with non-natural amino acids and unique chain pairings, allowing for the design of antibodies with novel functionalities that target difficult-to-reach or poorly immunogenic antigens. Phage display has been successfully used to target challenging proteins like CHIP, Gαq, and CS3D, which are difficult to target with traditional methods. Additionally, phage display is scalable, allowing for the production of large quantities of high-affinity binders for further testing and development.
Phage Display Technology Limitations
While phage display technology offers significant advantages, it also has several limitations that can impact its effectiveness in antibody discovery and therapeutic development.
One major challenge is amplification bias during the panning process. Since only a limited number of clones are selected in each round, rare antibody sequences may be underrepresented, which reduces the chances of detecting potentially valuable antibodies. This bias can hinder the identification of optimal binders, especially when targeting difficult or less abundant antigens.
Another limitation is the conformational discrepancy between antibodies on the phage surface and their chemically synthesized counterparts. Antibodies displayed on phages may not always fold correctly, especially larger or more complex antibodies, leading to non-functional or misfolded variants. This is particularly problematic for full-sized antibodies, which may not display properly on phages, reducing the selection of effective antibodies. While small peptides or antibody fragments, such as scFvs, are often displayed successfully, the ability to produce fully folded, full-length antibodies is still a challenge.
Library size is another constraint. The efficiency of bacterial transformation typically limits phage display libraries to around 10^10 to 10^11 variants, which may not be large enough to guarantee the identification of all possible binders for a given target. While alternative techniques like mRNA and ribosome display can generate larger libraries (10^14), they are distinct methods and do not fully address the limitations of phage display. For reference, the entire natural immune response can have as many as 10^18 antibody variants.
Additionally, phage display is typically performed in E. coli, which lacks the machinery to provide crucial post-translational modifications (PTMs) that can affect antibody functionality. For example, E. coli has been shown to preferentially express proteins rich in methionine and lysine and cannot fold complex immunoglobulins or provide critical PTMs like glycosylation and disulfide bonds. Although eukaryotic display platforms, like yeast or mammalian expression systems, can address this, they come with added complexity and challenges related to system optimization.
Phage display also faces issues with biased repertoires and potential loss of natural antibody pairing. Modifications to phage coat proteins to improve display or targeting can compromise the phage’s stability and infectivity. For instance, while changes to the phage tail protein can enhance specificity, alterations to the main coat protein might destabilize the phage, hindering its viability and limiting the display of antibodies.
Another limitation is related to the accessibility of target antigens. Effective binding requires that the target antigen be readily accessible on the phage surface. Small or hidden targets, such as those buried deep within a protein or in challenging epitopes, can be difficult to capture using phage display. Additionally, some targets may require extensive antibody engineering or structural modifications that phage display alone cannot easily address.
In therapeutic applications, phage display presents additional challenges. Antibodies developed using phage display may not always be well-tolerated by the immune system, particularly if they are derived from non-human sources. To reduce the risk of immunogenicity, humanization techniques are often necessary, which adds complexity to the development process. Even after humanization, phage display-generated antibodies can still present issues, including the potential for generating anti-drug antibodies (ADAs), which can reduce the efficacy of the therapy. For example, adalimumab, a fully human antibody produced via phage display, has been associated with neutralizing responses in as many as 89% of patients. This arises from challenges in replicating the full natural structure of antibodies, especially in the non-germline regions and due to variability introduced during affinity maturation.
While phage display is biased towards producing high affinity antibodies, high binding affinity does not necessarily equate to therapeutic efficacy. Even if an antibody has a strong affinity for its target, it may lack the biological activity required for clinical use. Because phage display is conducted in an in vitro environment, the selected antibodies are not known to perform well in the more complex, dynamic conditions of a living organism. The absence of post-translational modifications (e.g., glycosylation) and the lack of an immune system to select for ideal binders means that antibodies selected through phage display may not always exhibit the desired functionality in vivo.
Finally, targeting difficult epitopes remains a challenge. Phage display may struggle to identify antibodies against certain types of epitopes, such as those that are buried, conformational, or require specific post-translational modifications. These epitopes may not be well-represented in the phage display library, limiting the success of the approach for some targets. The inability to target complex antigens, such as multispanning membrane proteins, further highlights the method’s limitations in certain therapeutic areas.
The Future of Phage Display Technology
Phage display technology has evolved significantly over the past few years, with new advancements enhancing its capabilities and applications across drug discovery, vaccine development, and diagnostics.
Recent innovations include the creation of more diverse phage libraries, such as synthetic and yeast display libraries, which offer greater sequence diversity and improve results in protein interactions. The integration of next-generation sequencing (NGS) has revolutionized high-throughput screening, enabling faster and more accurate analysis of large phage display libraries.
Additionally, enhanced panning techniques such as high-throughput microfluidics systems allow for the isolation of high affinity antibodies more efficiently. Flow cytometry based single cell sorting or high throughput FACS also enhances the panning process by increasing the speed and efficiency at which individual phage clones with high affinity can be isolated.
Increasing attention is being given to the rational design of antibodies targeting specific epitopes on antigens. This approach combines structural data, such as crystal structures of the target or antibody fragments, with bioinformatics predictions based on amino acid sequences and modeling algorithms like Rosetta and AlphaFold. The rise of machine learning and structural-based approaches is leading to more targeted and efficient antibody discovery, exemplified by successful AI-based discovery of novel and therapeutic SARS-CoV-2 antibodies. Additionally, AI-based phage display antibody discovery has significantly improved immunogenicity issues of phage display antibodies with deep learning models predicting humanness. Albeit, immunogenicity can arise from several factors beyond humanness, including solubility, aggregation potential, cross-reactivity, and overall product stability. To address these complexities, researchers are working on models that predict both binding affinity and a “naturalness” metric.
To address the difficulty with finding biologically relevant antibodies for multispanning membrane proteins, such as ion channels, scientists have recently used kinetically controlled proteases as tools to identify potential antibody binding sites on disease-related proteins. By carefully guiding the proteases, disease relevant sites can be identified and used at the target antigen for phage display antibody development. For instance, this technique was used to develop antibodies for the TRPV1 ion channel, which was previously considered undruggable with antibodies, to discover conformationally sensitive antibodies. Similarly, techniques like hydrogen-deuterium exchange (HDX) epitope mapping can be used to identify difficult to target epitopes to use for phage display antibody discovery. These strategies highlight the importance of understanding the target’s structure, dynamics, and its role in disease.
Overcoming Limitations of Phage Display Antibody Discovery
One of the best ways to de-risk antibody discovery is to leverage diverse and complementary approaches.
Phage display enables the selection of high-affinity antibodies from large libraries by mimicking the natural immune response in vitro. In contrast, polyclonal sequencing offers a direct view of the entire antibody repertoire, capturing the outcomes of in vivo affinity maturation.
Together, these approaches provide a powerful strategy: phage display identifies high-affinity therapeutic candidates, while polyclonal sequencing uncovers the full diversity and specificity of the immune response, ensuring no valuable antibodies are missed.
Contact our scientists to learn about our antibody discovery services.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics