Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: February 13, 2025
What is the Major Histocompatibility Complex?
The major histocompatibility complex (MHC) is an essential component of our immune system, playing a crucial role in how our body recognizes and responds to foreign pathogens, including viruses, bacteria, and cancer cells.
The MHC consists of a set of genes, called the human leukocyte antigen (HLA) genes, that encode proteins located on the surface of most cells in the body. These proteins bind small fragments of peptides derived from pathogens and display them on the cell surface for recognition by immune cells, such as T-cells.
The MHC’s ability to distinguish between “self” and “non-self” is vital for maintaining effective immune surveillance and preventing harmful infections. If the MHC malfunctions, it can lead to various immune-related problems, such as impaired immune responses, autoimmune diseases, and increased susceptibility to infections or cancer.
Major Histocompatibility Complex Structure and Function
The MHC molecules are made up of two primary classes, namely class I and class II, that are encoded by more than 200 genes on chromosome 6. There are three class I α-chain genes, known as HLA-A, -B, and -C. There are also three pairs of class II α- and β-chain genes, called HLA-DR, -DP, and -DQ. These protein subunits then come together to form the final class I and class II MHC molecules that are anchored to the cell membrane.
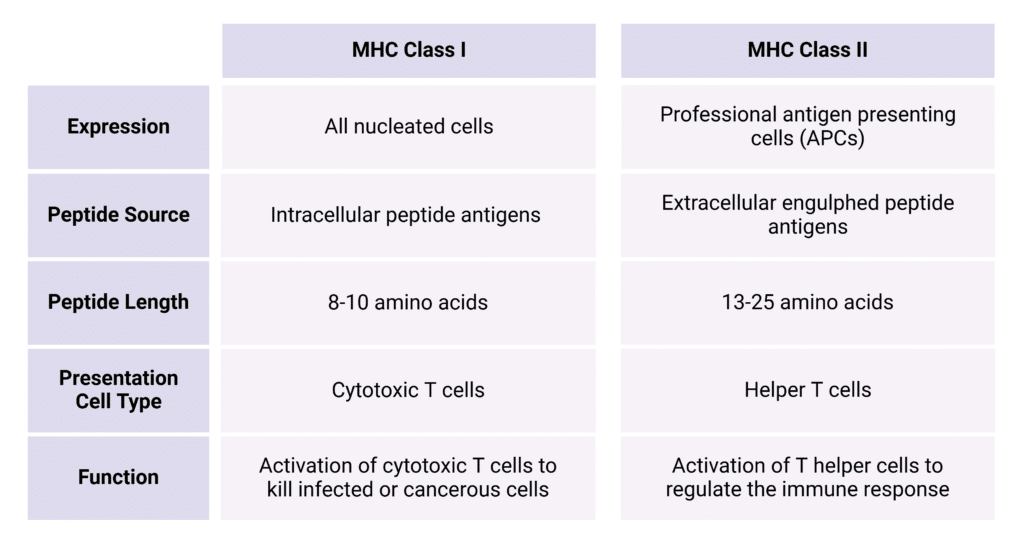
While the both classes of MHC serve to present peptide fragments to immune cells, each of the classes have distinct roles in the immune surveillance response. The type of immune cell they interact with, the type of cell they are expressed on, the kinds of peptides they present, and the final structure they form on the cell surface are all dependent on the class (Table 1).
Table 1: Summary of comparison between MHC Class I and Class II.
MHC Class I Structure and Function
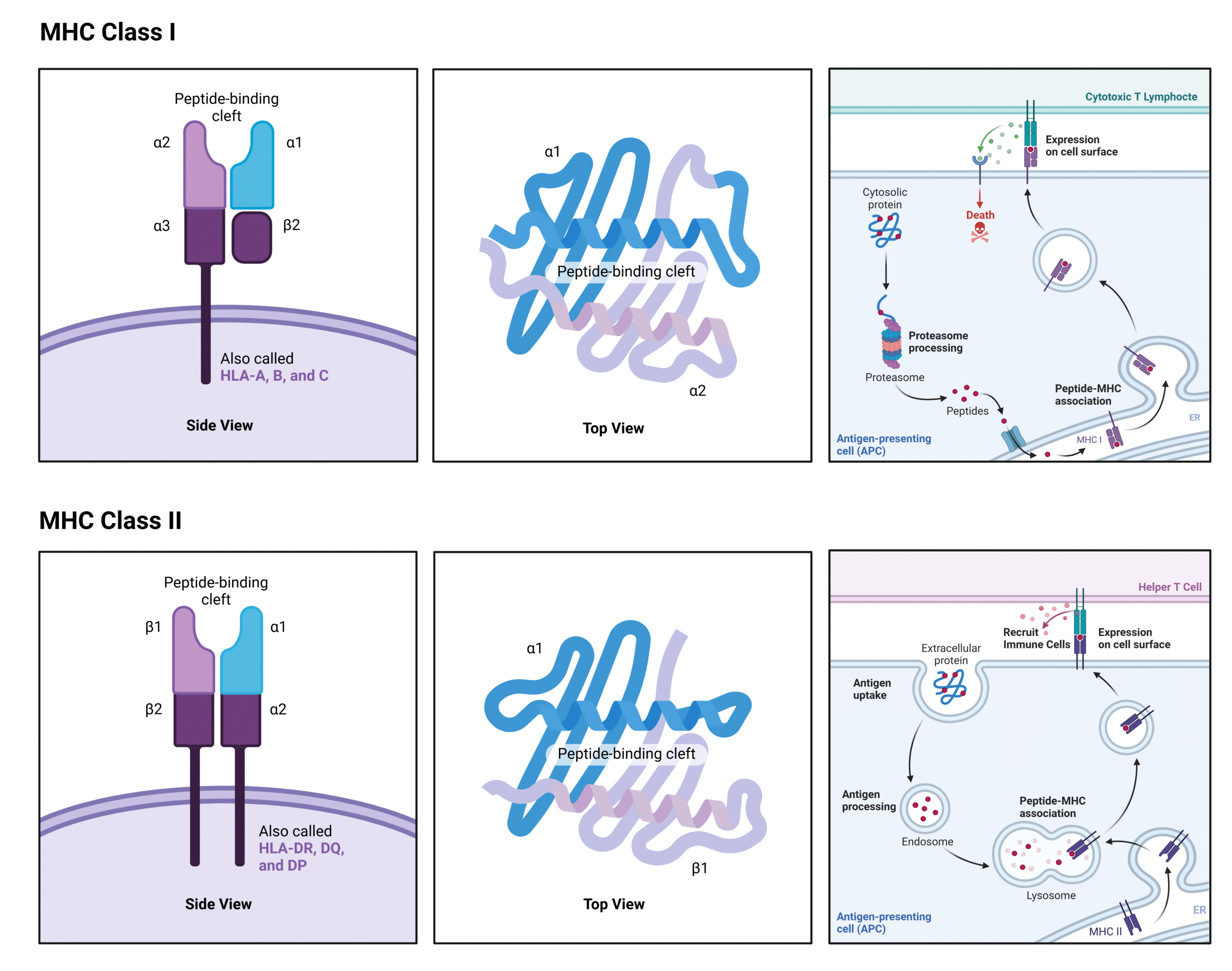
MHC class I molecules are present on nearly all nucleated cells in the body. They consist of an alpha chain, which anchors the molecule to the cell membrane, and a smaller protein called beta-2 microglobulin, which helps stabilize the structure (Figure 1). The alpha chain forms a groove that binds small peptide fragments, typically 8-10 amino acids in length. This groove is where pathogen-derived peptide antigens are presented for recognition by cytotoxic T lymphocytes, which are responsible for killing infected cells.
Class I antigen presentation begins with the proteasomal degradation of intracellular proteins into peptides. These peptide fragments then bind to MHC class I molecules in the endoplasmic reticulum (ER), forming a receptor-peptide complex that is transported to the cell surface. At the cell surface, the peptide antigen is presented to cytotoxic T cells. If the presented antigen is recognized as foreign, the cytotoxic T cell triggers an immune response that leads to the destruction of the infected cell.
A key feature of MHC class I molecules is their ability to present peptides derived from intracellular proteins, which can include both self-proteins and pathogen-associated proteins, such as viral or mutated cancer proteins.
MHC Class II Structure and Function
MHC class II molecules are primarily expressed on antigen-presenting cells (APCs), such as dendritic cells, macrophages, and B cells. These molecules consist of two chains—the alpha and beta chains—which together form the peptide-binding groove. This groove binds longer peptide fragments, typically 12-24 amino acids in length (Figure 1). Unlike MHC class I molecules, the groove of MHC class II opens at both ends, allowing it to accommodate longer peptides.
Class II antigen presentation begins with the engulfment of extracellular proteins, which are then degraded in the lysosome. The MHC class II molecules are transported from the ER to the lysosome, where they bind to the peptide fragments. The receptor-peptide complex is then moved to the cell surface, where the peptide is presented to helper T cells. The helper T cells recognize the peptide using their T cell receptors (TCRs), become activated, and release cytokines to initiate an immune response, including the recruitment of macrophages.
A defining feature of MHC class II molecules is their role in presenting extracellular pathogen-derived peptides from engulfed pathogens, such as bacteria and fungi.
Figure 1: Illustration of the Structure and Function of MHC class I and class II molecules.
Major Histocompatibility Complex Adaptability and Evolution
The immune system is in a constant arms race with pathogens, driven by mutation, genetic diversity, selection, and recombination. As a result, the immune system has evolved to be highly adaptable, enabling it to protect the body against a wide array of pathogens. The adaptability and evolution of the major histocompatibility complex (MHC) are primarily shaped by two key factors: polymorphism and polygeny.
The MHC gene is polygenic, meaning it contains multiple different MHC class I and class II genes that interact additively to determine the final receptor product. This process, also known as codominance, ensures that the protein products of each gene are expressed and capable of presenting antigens to T cells. Because of this polygeny, individuals typically express at least three different MHC class I molecules and three to four class II molecules on their cells.
The MHC genes are among the most polymorphic in the genome, with over 200 variants of most MHC class I and II genes. These genes also exhibit the largest number of single nucleotide polymorphisms (SNPs) linked to nearly 500 traits and diseases. In fact, the MHC region remains one of the least characterized parts of the human genome. Due to their high polymorphism, most people express at least six different MHC class I molecules and eight different MHC class II molecules on their cells. The likelihood of inheriting two identical MHC genes is negligible, further increasing the genetic diversity and the range of pathogen-derived peptides the MHC can present.
Moreover, the protein products from a single MHC gene can differ by up to 20 amino acids, making each protein distinct. This variation, which is concentrated in the peptide-binding groove, directly influences antigen presentation. The amino acid residues in the peptide-binding groove, known as the sequence motif, dictate the binding specificity of the MHC for different peptides. This variability enhances the diversity of antigen presentation, allowing the immune system to recognize and respond to a broad spectrum of pathogens. Understanding these sequence motifs can aid in the development of therapeutics, such as vaccines, by identifying which pathogenic peptides are likely to bind to specific MHC molecules.
Major Histocompatibility Complex Dysfunction
The MHC is one of many mechanisms contributing to the immune system outflanking the evasive maneuvers of pathogens. However, like most things in life, the MHC is not perfect and sometimes pathogens are able to evade the immune system by avoiding MHC detection.
There are two primary strategies through which pathogens can avoid recognition by the MHC. The first is through mutations that render the pathogen’s proteins unrecognizable to the MHC. A well-known example of this is Epstein-Barr virus (EBV), where certain viral strains have mutations in the peptide epitopes presented by the MHC. These mutated peptides no longer bind effectively to the MHC, allowing the virus to evade detection by T cells. The second strategy is for pathogens to directly interfere with MHC expression or function. Some viruses, like adenoviruses, produce proteins that bind to the MHC in the ER, blocking their transport to the cell surface and effectively downregulating MHC function. Similarly, both human immunodeficiency virus (HIV) and herpes simplex virus (HSV) downregulate MHC expression to avoid T cell detection and immune surveillance.
MHC class I molecules also play a crucial role in identifying and eliminating abnormal cells, such as cancerous ones, by presenting mutated self-peptides to cytotoxic T cells. To survive and proliferate, cancer cells must develop mechanisms to evade MHC-mediated recognition. Certain cancers have been shown to mutate to lose MHC class I expression entirely, allowing them to escape T cell detection. Furthermore, specific MHC polymorphisms have been linked to susceptibility to certain cancers, such as lymphoma and melanoma. Mutations in MHC class I can also confer resistance to various types of immunotherapy, making cancer treatment more challenging.
This raises an interesting question – why haven’t humans evolved to have an even greater number of MHC genes to gain an upper hand in the immune arms race? The answer likely lies in the balance between immune protection and self tolerance. Increasing the number of MHC genes would not only expand the range of pathogen-derived peptides presented, but also self-peptide presentation. This could undermine self-tolerance mechanisms and potentially lead to autoimmune diseases, where the immune system attacks the body’s own tissues. For instance, certain HLA-DR alleles in MHC class II molecules are linked to autoimmune diseases like rheumatoid arthritis. Similarly, specific HLA-DQ and HLA-DR alleles are associated with an increased risk of developing Type 1 diabetes. On the flip side, certain MHC alleles can impair MHC function altogether, contributing to immunodeficiencies, as seen in Bare Lymphocyte Syndrome (BLS).
Major Histocompatibility Complex and Immunopeptidomics Sequencing with Rapid Novor
MHC research has historically been challenging due to the complexity and diversity of MHC molecules across individuals. Sequencing the MHC proteins, rather than just their encoding genes, offers a deeper insight into their function and interactions.
Rapid Novor’s mass spectrometry-based protein sequencing platform can sequence proteins de novo, meaning it does not require prior knowledge of the peptide amino acid sequences or sequence databases. This technology can allow researchers to observe MHC functionality in response to different pathogens or therapeutic interventions, accelerating the development of immune therapies and vaccines.
Additionally, Rapid Novor can perform de novo MHC peptide sequencing to rapidly identify and analyze MHC-bound peptides. Adding de novo sequencing to immunopeptidomics studies may give a clearer view of immune responses to pathogens, cancer, and therapeutic interventions. Such sequencing is powered by our Novor AI algorithm, which is currently available for license through Bruker: PaSER™ Novor. If you would like to license Novor AI for your immunopeptideomics studies using Thermo Orbitrap or other mass spectrometers, or if you would like Rapid Novor to perform such sequencing for you, please contact our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics