Written by:
Written by:
Vanessa Yoon Calvelo, PhD
Published: June 6, 2023
Updated: July 7, 2024
Introduction
Antibodies offer a vast range of configurations for therapeutic applications, including antibody drug conjugates (ADCs), multi-specific formats and engineered protein-binding fragments. The process of drug discovery for mAbs is different from small-molecule drugs and requires extensive discovery, characterization, and optimization efforts due to the complexities of antibody diversity, structure, and function.
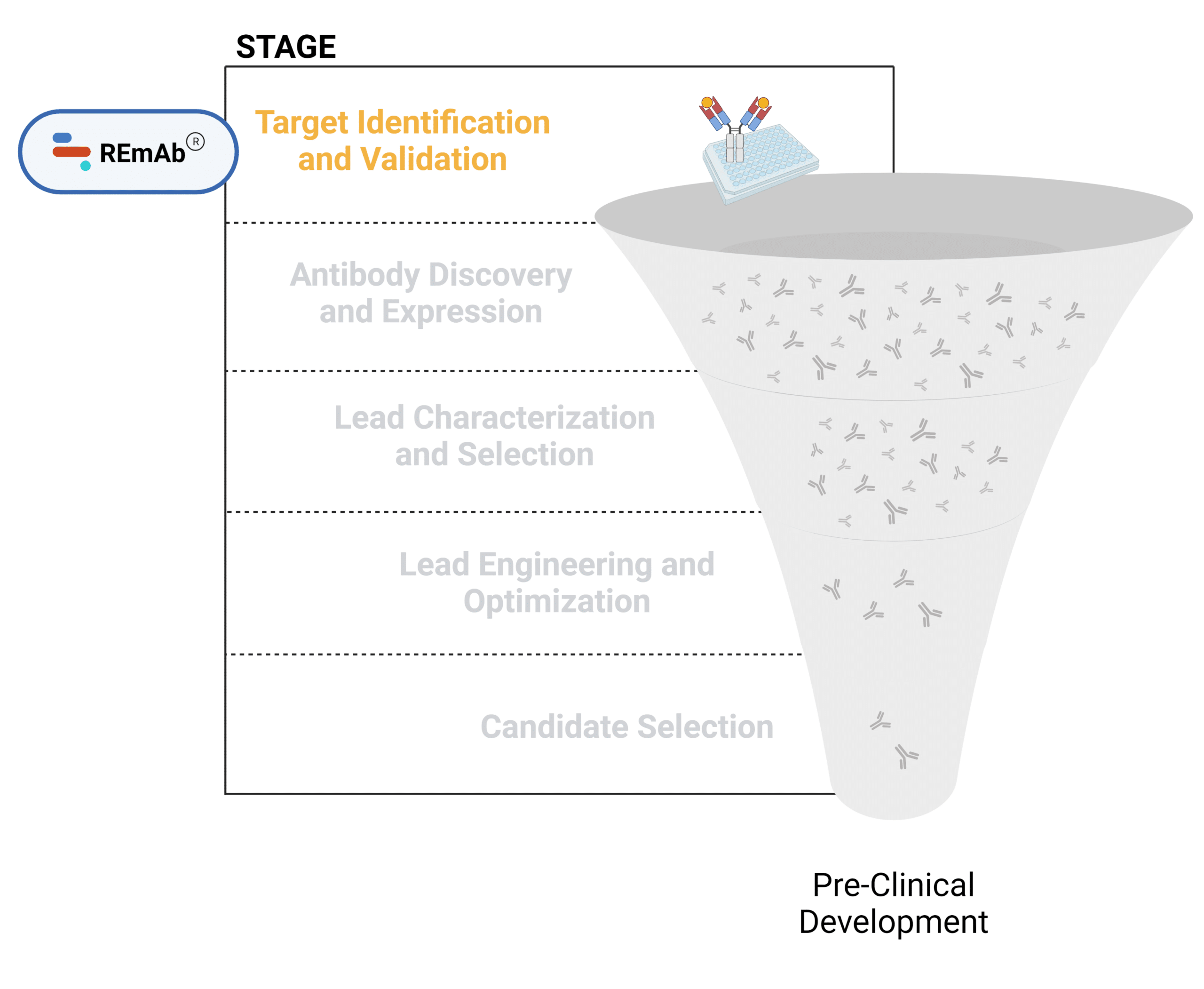
The mAb therapeutic discovery process can be divided into 5 stages (Figure 1):
- Target discovery and validation
- Antibody discovery and expression
- Lead characterization and selection
- Lead engineering and optimization
- Candidate selection + downstream characterization
Below, we dive into the early discovery and development phase and provide a list of our resources available that are associated with each stage involved in discovering therapeutic antibodies (Figure 1).
Early Discovery and Development of Therapeutic Antibodies
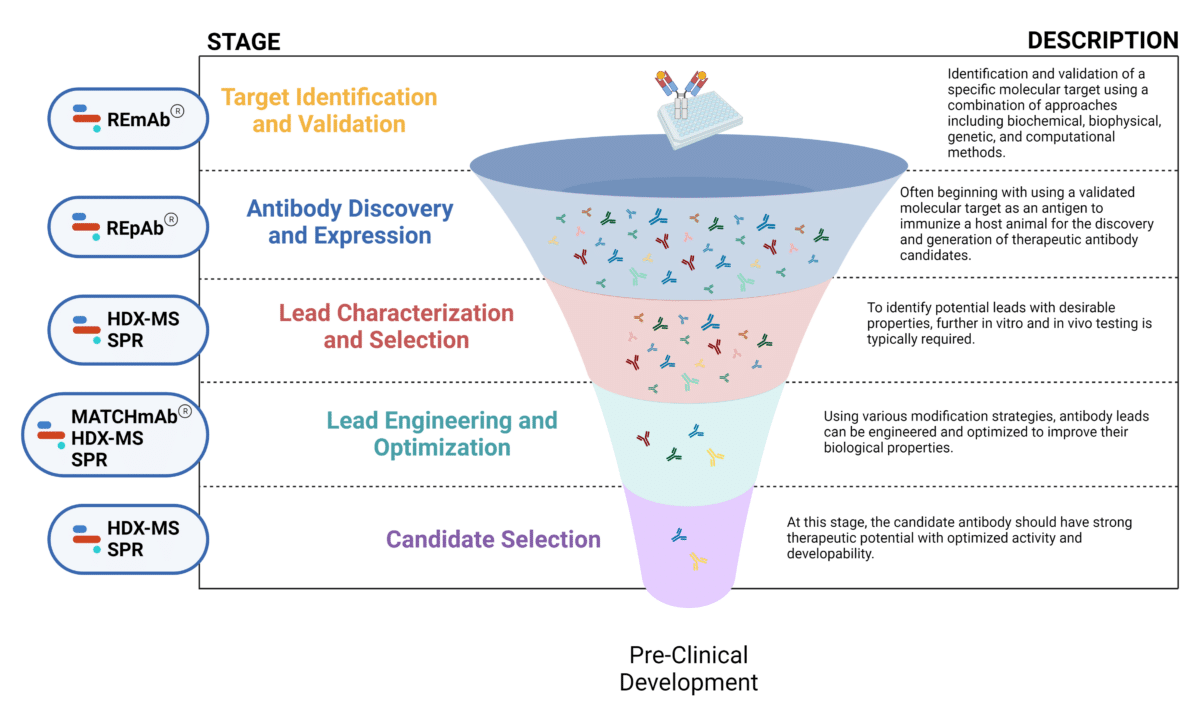
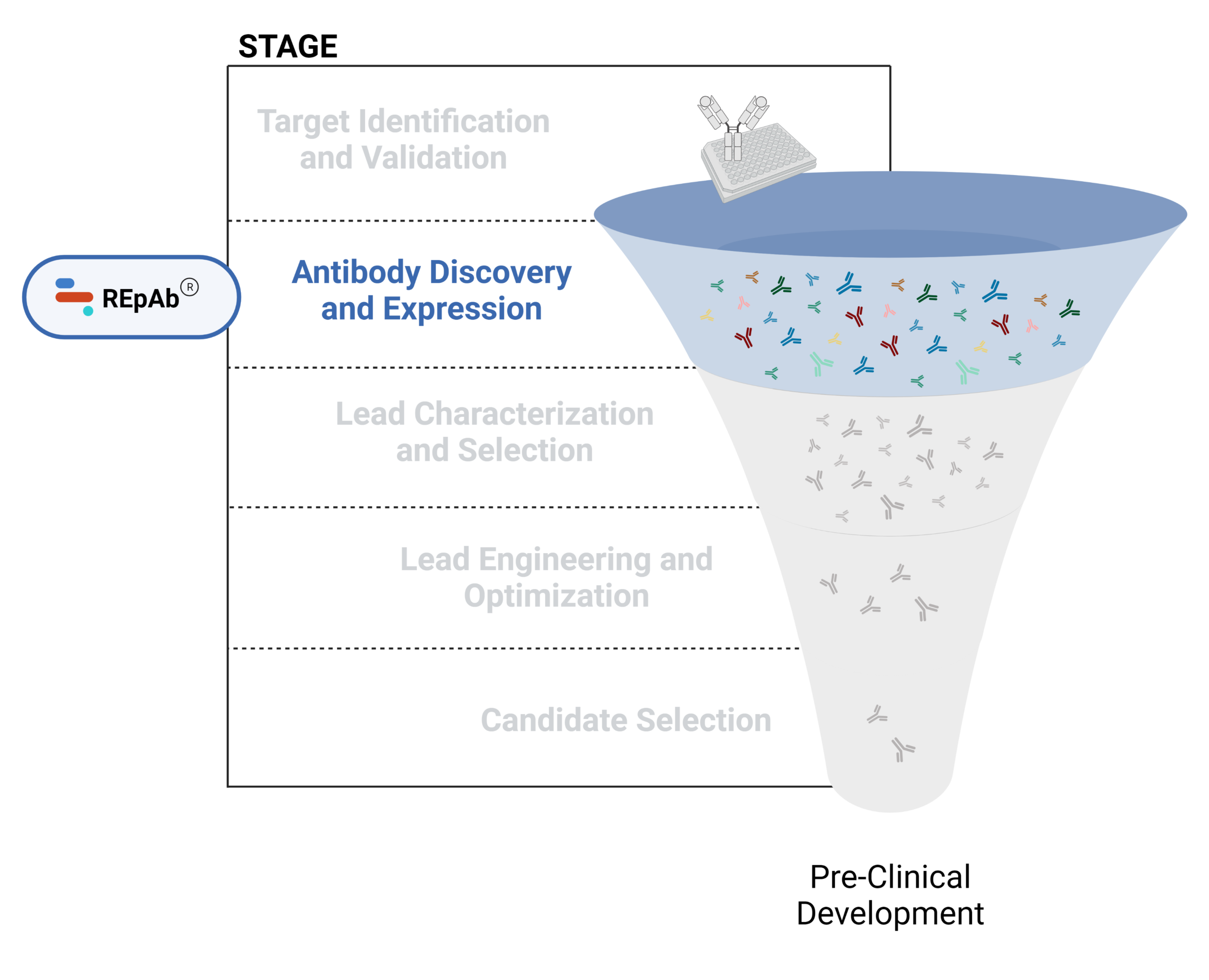
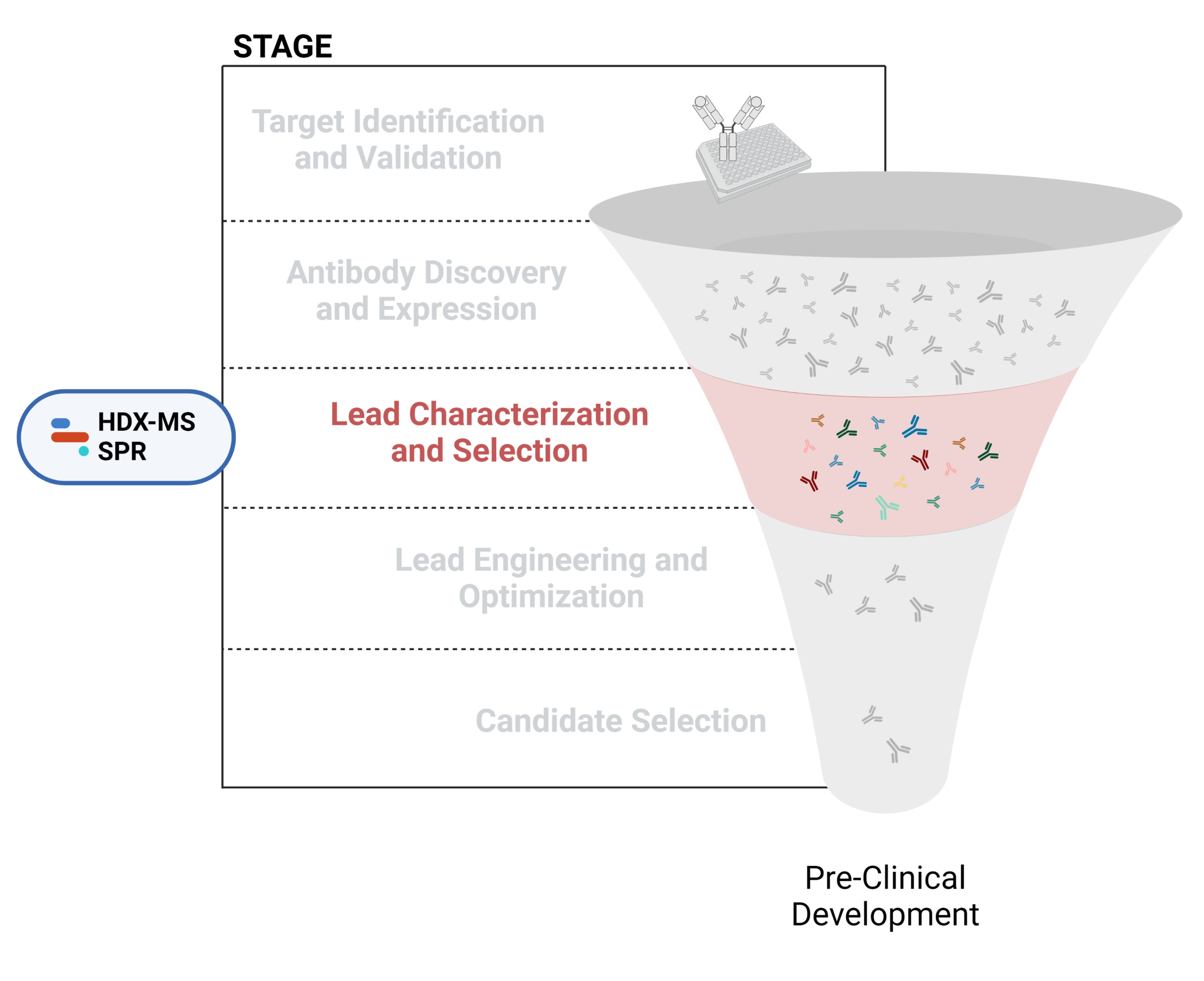
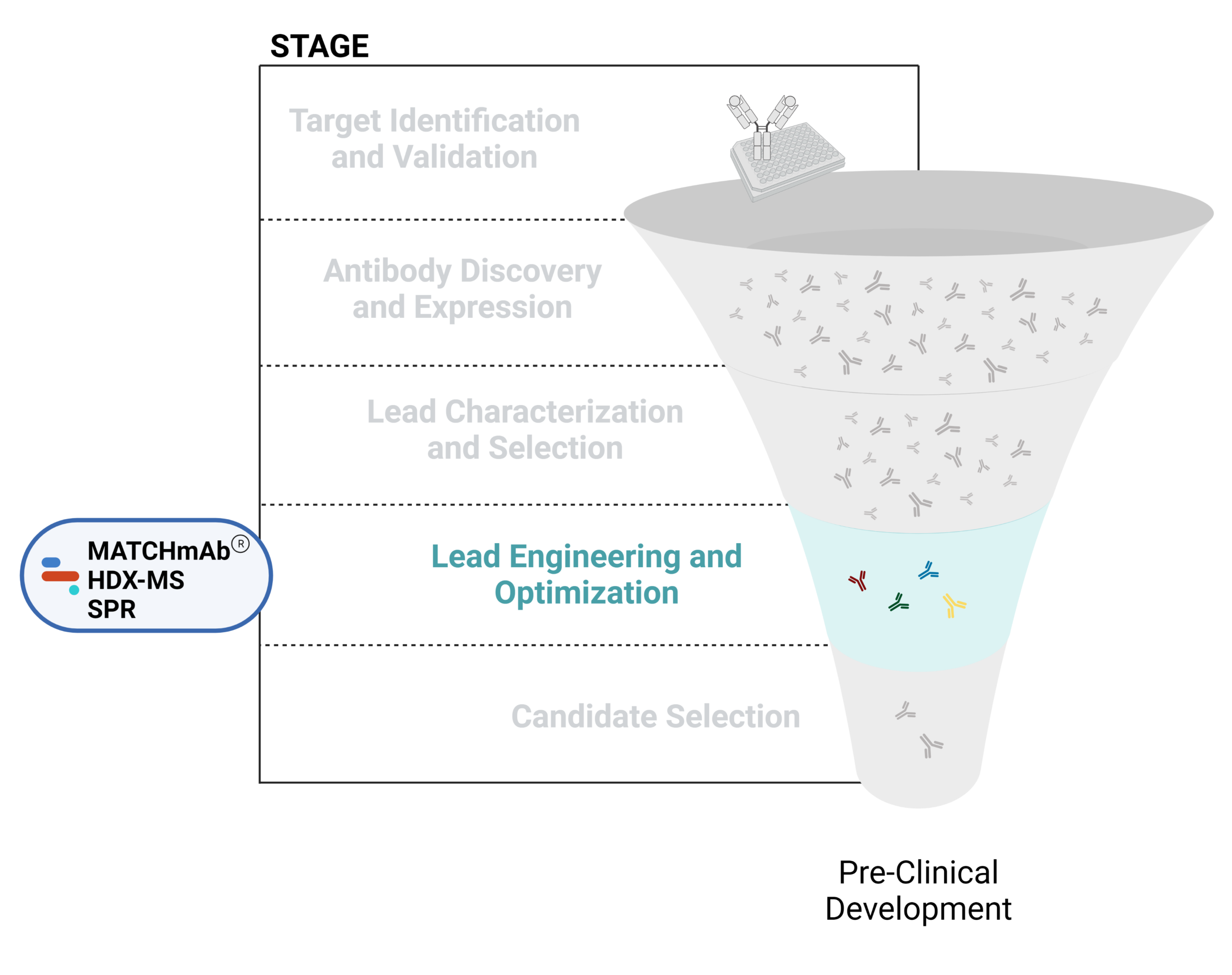
Figure 1. Five stages of the early discovery and development phase in the drug development pathway.
Target Identification and Validation
The primary goal of target identification and validation in antibody discovery processes is to pinpoint and confirm the specific molecules, such as proteins or receptors that play a crucial role in disease processes. These targets must be highly specific to minimize off-target effects and enhance therapeutic efficacy.
The challenges in this process include the complexity of biological systems, where potential targets are involved in multiple cellular processes and can be expressed ubiquitously across diseased and healthy tissues and cells. Additionally, ensuring that the identified target is not only disease specific, but also accessible to antibody binding and modulation adds another layer of complexity. Depending on the target, there may not be tool antibodies available to help study the biological pathway related to the target. Finding and validating tool antibodies can be time consuming.
Advanced techniques, such as proteomics, genomics, and bioinformatics are essential to overcome these challenges, providing a comprehensive understanding of target biology and validating its relevance in disease contexts.
Check out our resources below on de novo protein sequencing and the applications for target identification and validation.
- Understanding and validating tool antibodies and reagents used to study the target: Antibody Validation and its Use Cases
- Understanding competing IP interests about the target: Secure Full Antibody and Target IP Protection with Next Generation Protein Sequencing
- Protein Structure and How to Study It
- Next Generation Protein Sequencing in Veterinary Medicine and Industry
Antibody Discovery and Expression
Antibody discovery involves a combination of in vitro and in vivo methods, beginning with antigen immunization for the generation of therapeutic antibody candidates. The primary goal is to identify highly specific antibodies that effectively target disease-related antigens and are suitable for further development. The main challenges reported in antibody discovery include capturing a diverse antibody repertoire, functional screening, and preventing developability challenges downstream.
Technologies for antibody discovery include hybridoma generation, B cell sequencing, display technologies, and de novo polyclonal antibody sequencing. Computational (AI-based) approaches for antibody discovery are gaining traction, and are most often employed to augment experimental approaches.
Learn more about antibody discovery methods and our mass spectrometry-based platform for antibody discovery in our latest articles below.
Lead Characterization and Selection
To identify potential leads with desirable properties, further in vitro and in vivo testing is typically required, aiming to find candidates with optimal efficacy, safety, and manufacturable profiles for clinical development This involves thoroughly assessing binding specificity, affinity, stability, and functional activity against the target antigen. Additionally, scalability in production and formulation stability are critical factors that must be considered to ensure the successful transition from laboratory to clinical applications.
Characterization of structural, physicochemical, immunochemical, and functional properties is essential in evaluating and selecting lead candidates for downstream development. Advanced analytical techniques, such as HDX-MS and SPR, play a crucial role in overcoming these challenges, providing comprehensive insights into antibody characteristics and facilitating informed decision-making in therapeutic development.
Please refer to our latest resources below for further information on antibody characterization.
- Immunochemical Characterization of Therapeutic Antibodies
- Functional Characterization of Therapeutic Antibodies
- What is HDX-MS Epitope Mapping?
- Protein Characterization by HDX-MS
- Surface Plasmon Resonance Spectroscopy
- Characterizing Biomolecular Interactions with Surface Plasmon Spectroscopy
- Epitope Binning With SPR For Antibody Drug Discovery
Lead Engineering and Optimization
Using various modification strategies, antibody leads can be engineered and optimized to improve their biological properties including effectiveness, affinity, and immunogenicity. This process can involve modifying antibody structures to achieve desired attributes, such as reduced immunogenicity, increased half-life, and improved tissue penetration. It can also involve creating bispecific and multispecific antibodies, antibody-drug conjugates, CAR-T cells, or other mAb-based therapeutics like PROTACs.
Challenges in antibody engineering and optimization include maintaining a balance between enhancing desired properties and preserving the functional integrity of the antibody. Modifications, such as linker domains, can sometimes lead to unintended consequences, such as loss of binding affinity, increased immunogenicity, or higher aggregation propensity.
Engineering efforts have focused on expanding the applications of antibody therapeutics by generating humanized antibodies, antibody fragments, multispecifics, and antibody-drug conjugates.
Check out our articles below to learn more about the applications of proteomics-based technologies in antibody engineering and optimization efforts.
- The Hunt for Novel Therapeutics Through Antibody Engineering
- Rational Antibody Design and Engineering with Next Generation Protein Sequencing
- The Landscape of Bispecific and Multispecific Antibodies
- Antibody-Drug Conjugates as Anti-Cancer Therapeutics
- Cell Specific Protein Degradation with Antibody Conjugated PROTACs
- Chimeric Antigen Receptors and T Cells – CAR-T
Candidate Selection
Once the antibody leads have undergone the four stages of early discovery and development mentioned above, these antibodies are either selected or not selected for further rigorous characterization. As the final one or two candidates, these antibodies should have strong therapeutic potential with optimized activity and developability, which are chosen for pre-clinical development. Here, the focus shifts from discovery to preparing for production.
Challenges in this process include the complexity of thoroughly characterizing antibodies due to their large size and intricate structures. Maintaining consistency and quality across different production batches is critical, as any variation can impact efficacy and safety. Additionally, regulatory agencies require extensive data and stringent compliance with guidelines, which can be resource-intensive and time-consuming.
Advanced analytical techniques, such as mass spectrometry are essential for detailed characterization. These methods provide in-depth insights into the antibodies’ properties and behaviors, facilitating the identification of any potential issues early in the development process. Ensuring robust and reproducible characterization data is crucial for successful regulatory submissions and subsequent clinical development.
Check out our articles below to learn more about the applications of LC-MS based antibody characterization and candidate selection:
- Navigating Antibody Characterization through LC-MS
- Why do Post-Translational Modifications Matter?
- What is Peptide Mapping?
- The Basics of Protein and Antibody Glycosylation
- Modifying Antibody Functions Through Glycan Engineering
- Antibody Production: A Guide to Choosing a Recombinant Expression System
For a more detailed discussion on the therapeutic antibody discovery process, please refer to our article: Therapeutic Antibody Discovery: From Target to Candidate.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics