Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: November 6, 2024
Why Humanize Antibodies?
Antibodies are integral components of our immune system and have become essential tools in therapeutic development, particularly for treating diseases like cancer, autoimmune disorders, transplantation, cardiovascular disease, and infectious diseases. Since the approval of the first therapeutic antibody in 1986, over a hundred novel antibodies have gained approval for therapeutic use in humans. Antibodies now represent half of all pharmaceutical sales, with five of the top ten selling drugs being antibodies, highlighting their critical role in therapeutics.
Given that antibody therapeutics are typically produced in non-human sources, the journey from discovery to clinical application often encounters significant barriers, such as immunogenicity. The initial use of muromonab, a mouse derived monoclonal antibody, highlighted these barriers, such as triggering human immune responses that lead to the production of neutralizing antibodies, extremely short half-lives, and inadequate human antigen binding or immune response activation. These barriers have led to the practice of antibody humanization, a process aimed at enhancing the antibodies compatibility with human systems. Although humanization improved safety and efficacy, barriers persist after humanization and resolving these outstanding challenges is at the forefront of innovation in antibody therapeutics.
What is Antibody Humanization?
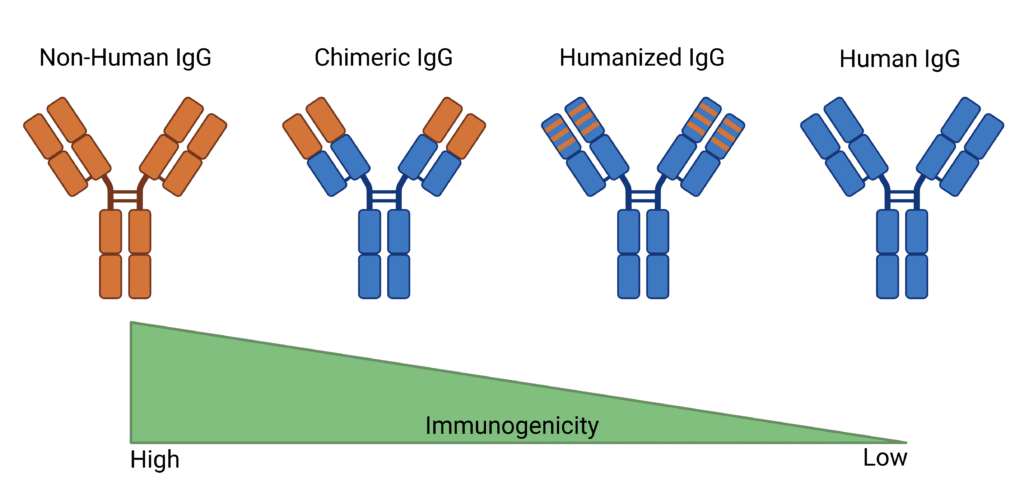
Antibody humanization is a process invented to modify antibodies that have been synthesized in non-human sources (often mice or other species) to make them more similar to human antibodies. The humanization process involves transferring essential non-human amino acid sequences onto a human antibody framework to humanize the antibody while retaining the disease targeting binding properties. This process may create human chimeras, transferring larger non-human protein sections, or focus on selectively altering variable regions while preserving crucial non-human sequences for antigen binding (Figure 1). Techniques like grafting, where the complementarity-determining regions (CDR) are transferred to a human antibody framework, are commonly used in this process. The more humanized the antibody, the less likely it is to cause negative side effects, resulting in a safer therapeutic option.
However, it is important to strike a balance, as excessive humanization can lead to a loss of therapeutic function. This balancing act between safety and efficacy contributes to the lengthy development process. Most therapeutics have historically been humanized mouse antibodies due to earlier limitations in technology and the challenges of generating fully human antibodies. Humanized antibodies were a compromise, designed to retain the functionality of mouse antibodies while reducing their immunogenicity in humans. As technology continues to advance, the trend is moving towards the use of fully human antibodies for better therapeutic outcomes.
Figure 1: Comparison of Non-Human, Chimeric, Humanized, and Human IgG Antibodies Immunogenicity.
Antibody Humanization Techniques
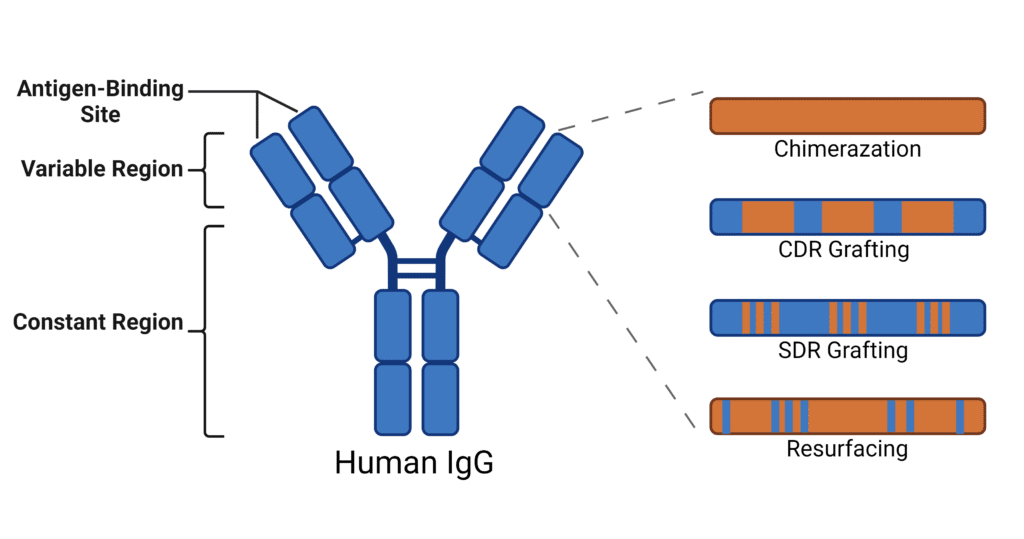
Antibody humanization techniques involve various experimental approaches. Described here are the most prominently used strategies (Figure 2):
- Chimerization: The first chimeric antibody, abciximab, received FDA approval in 1994, and was created by fusing the variable regions of a non-human antibody with the constant regions of a human IgG antibody. This method combines the binding properties of the original antibody while utilizing the more compatible human constant domains. Although improved, chimeras still produced a great deal of challenges when it came to adverse effects like immunogenicity.
- CDR Grafting: To further decrease the immunogenicity of chimeras, CDR grafting was designed to decrease the amount of non-human sequences while maintaining the antigen-binding specificity of the original antibody. This involves transferring the CDR loops from a non-human antibody (often from a mouse) onto a human antibody framework. However, after construction, these humanized antibodies have to undergo extensive testing to ensure they retain their binding affinity and functionality. Often, CDR grafting requires additional changes to the framework, such as reversing mutations back to the non-human residue, to rescue function.
- SDR Grafting: To reduce the still-prevalent immunogenicity of CDR grafting, specificity-determining residues (SDR) grafting was designed to remove even more non-human sequences. To do this, the structure of antibody-antigen complexes are used to determine specific residues involved in antigen binding and graft those onto the human antibody framework. Although this method improves immunogenicity, it poses several challenges such as loss of specificity, functional impairment, and complex optimization.
- Resurfacing: Resurfacing is a technique used to humanize antibodies by modifying only the surface exposed residues. It involves analyzing the surface amino acids in the variable regions of murine and human antibodies, revealing the residues that have the potential to cause an immune response and convert them to human equivalents. It relies on the assumption that buried residues will not cause immunogenicity issues.
Figure 2: Depiction of Antibody Humanization Experimental Approaches.
Current Challenges of Antibody Humanization
For nearly 40 years, the development process of therapeutic antibodies has continually evolved, refining humanization strategies to enhance the pipeline for new treatments. Despite these advancements, significant challenges remain for humanized antibodies, which can still provoke immune responses due to residual non-human elements. A case in point is tocilizumab, a humanized anti-IL-6 receptor antibody that has been known to trigger immune reactions in some patients. Additionally, clinical data showed significant anti-drug antibody (ADA) occurrence in one of the most well-known humanized antibodies, Trastuzumab, that is used to treat HER-2 positive breast cancer.
Recent advancements in predictive algorithms and deep learning models, such as Rosetta, aim to improve the humanization process by using massive and diverse antibody sequence libraries. Several companies, such as MAbSilico, PipeBio, and ENPICOM, have developed their own platforms that streamline antibody humanization efforts by calculating properties such as immunogenicity, specificity, affinity, and manufacturability. In general, computational tools may decrease the length of trial-and-error during antibody humanization. However, limits to size and quality of training data can introduce unseen biases. Experimental evidence should be part of AI guided humanization efforts: AI focuses the experimental efforts, and experimental evidence is given back to the algorithms for refined predictions.
While achieving a close resemblance to human antibodies and maintaining binding affinity are promising indicators of successful humanization, it is crucial to assess the reliability of different humanness scoring methods. Various computational tools evaluate humanness in diverse ways, and attaining a higher humanness score does not necessarily correlate with reduced immunogenicity. Approaches, such as Hu-mAb, have further enhanced humanness scoring, achieving high classification accuracy in distinguishing human from non-human sequences and correlating higher humanness scores with lower immunogenicity. Platforms like BioPhi have also been developed for more precise humanization. Although these computational tools can predict the likelihood of immunogenicity, the correlation with actual anti-drug antibody (ADA) responses is generally weak.
Immunogenicity can arise from several factors beyond humanness, including solubility, aggregation potential, cross-reactivity, and overall product stability. To address these complexities, researchers are working on models that predict both binding affinity and a “naturalness” metric. This concept of naturalness is closely related to both the developability of the antibody and its immunogenicity. By optimizing for both binding affinity and naturalness, researchers may mitigate some of the persistent challenges associated with antibody humanization. It is important to note that immunogenicity can also be affected by patient-specific factors, such as immune status and HLA alleles, which impact the likelihood of anti-drug antibody (ADA) formation. Additionally, more convenient administration routes like subcutaneous injection can increase the risk of aggregation and ADAs due to higher concentrations of the antibody.
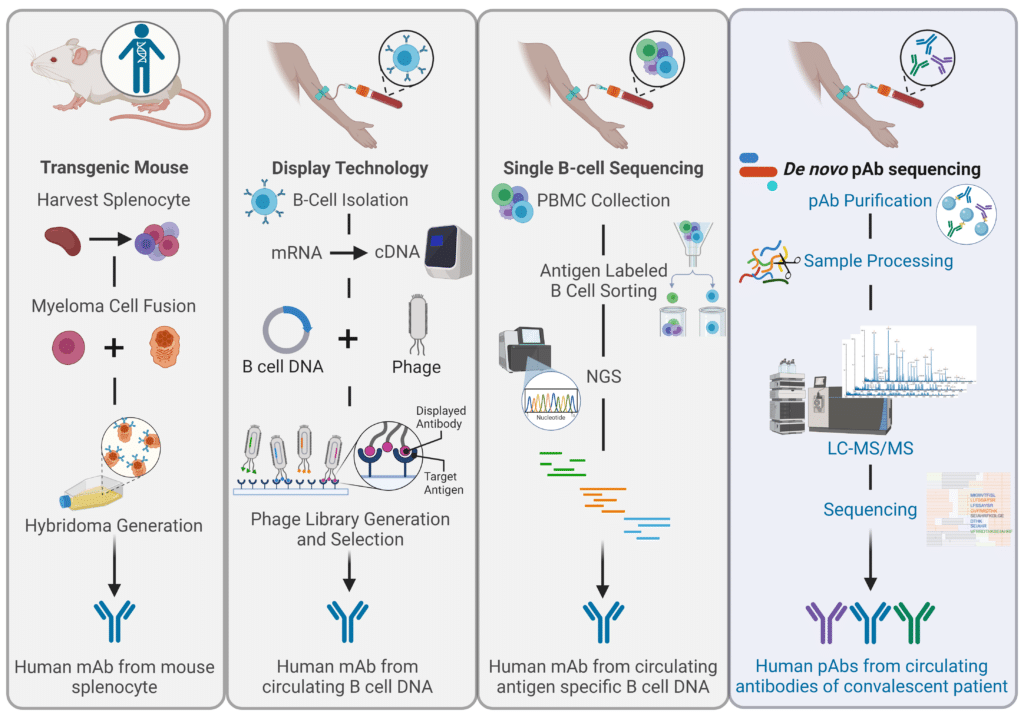
In response to these ongoing challenges with humanization, the industry has turned to fully human antibodies, which are generated using techniques such as transgenic animal models (Figure 3). The goal of these approaches is to eliminate immunogenicity entirely. However, even genetically human antibodies produced in non-human systems can be perceived as “unnatural” by the human immune system due to non-human protein structure, foreign PTMs such as glycosylation patterns, and size or complexity variations. Overall, the current understanding of “humanness” is limited, as genetically human antibodies can still trigger ADAs, influenced by factors like genetic diversity. For example, Golimumab, a fully human anti-TNF antibody synthesized in genetically modified mice, resulted in 16% of patients developing ADAs. In fact, a study surveyed humanized antibody therapeutics and found that there was no direct correlation between percent humanness and incidence of ADAs. Although unexpected, this finding is consistent with the complexity of immune pathways and further demonstrates that immunogenicity is multi-factorial. The authors were quick to note that the lack of correlation does not imply that antibody candidates originating from non-human species do not need humanization before clinical development. Despite weak scientific evidence supporting the need to improve humanness and naturalness in therapeutic antibodies, these efforts are still prioritized due to the high risks and costs of drug development, where even small improvements can significantly enhance a drug’s chances of success.
The Future of Human Antibody Therapeutics
The ongoing issues with humanization has led to fully human monoclonal antibodies becoming the gold standard for antibody therapeutics. Fully human antibodies can be generated through in vitro display techniques, such as phage display technology, that use high-throughput screening of large, diverse antibody libraries (Figure 3). However, antibodies created ex vivo lack the screening for polyreactivity that occurs through natural tolerance mechanisms, and they may not retain the necessary biophysical properties, such as isoelectric point (pI) or hydrophobicity. Additionally, these genetically human antibodies continue to elicit ADA production. This challenge likely arises from difficulties in accurately capturing the humanness of non-germline regions and the variability introduced by somatic hypermutation and V(D)J recombination during affinity maturation. For example, adalimumab, a fully human antibody produced via phage display, has been associated with neutralizing responses in as many as 89% of patients.
Based on these findings, scientists established that antibodies generated through the natural human immune response are a safer and more effective way to discover and develop therapeutics antibodies. Given this, B cell sequencing was developed as a method to isolate antibodies straight from convalescent patients. However, B cell sequencing poses its own challenges since it does not capture the full diversity of the antibodies circulating in the blood. Since single B-cell sequencing is limited to approximately 10,000 cells at a time, they often fail to effectively identify the most prominent or critical antibodies needed to combat specific pathogens. This gap can hinder understanding of which candidates will perform best in therapeutic settings. In the end, immune protection depends on the antibodies that are present in the serum, not the circulating B cells.
To circumvent this, recent research in Nature Communications demonstrates the use of de novo polyclonal antibody (pAb) sequencing to bypass the need for reference databases. By isolating antibodies from the blood of individuals who recovered from infections, this method taps into naturally occurring human antibodies that are compatible with the human immune system. These antibodies are then sequenced using tandem mass spectrometry (MS/MS) to create pAbs with high specificity and affinity, ideal for therapeutic use. For example, Rapid Novor used this technology to generate recombinant pAbs from a Moderna Spikevax vaccine, which showed similar or improved binding affinities and neutralizing capabilities against the target antigen.
Figure 3: Comparison of Techniques to Generate Fully Human Therapeutic Antibodies.
Human Antibody Discovery From Human Serum
Discovering antibodies directly in humans can address key challenges in the humanization process, such as reducing the risk of immune responses, optimizing therapeutic efficacy, and ultimately accelerating the therapeutic development pipeline.
Rapid Novor’s REpAb® technology sequences polyclonal antibodies (pAbs) using the proteins sourced directly from human blood samples, circumventing the need for lengthy humanization strategies. These isolated pAbs have already undergone affinity maturation and screening by the human immune system, making them a more natural starting point for developing effective antibody therapeutics.
Using mass spectrometry, REpAb® antibody discovery service reveals unique antibodies often missed by traditional B-cell sequencing.
Contact our scientists today to discuss your antibody discovery project.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics