Genya Gorshtein, MSc
Published: February 17, 2023
The Drug Development Pathway
The four phases in the drug development pathway are:
- Early discovery and development
- Clinical testing and trials
- FDA review
- Post-market research & monitoring
The development process of a new medicine across all therapeutic areas can take anywhere between ~12-15 years. The complexity of drug development has increased in the past few decades, requiring a phase of preclinical development, investigational new drug (IND) application, and complete clinical testing before submission for marketing approval from major regulatory agencies (ie. FDA, EMEA).
In addition to the lengthy process, pharmaceutical companies face challenges in profitability and growth as the number of truly innovative new medicines that are approved by regulatory agencies continues to decrease. The average cost for these companies to bring a new medicine to market is estimated to be ~$1.8 billion, and is rising rapidly. With these exorbitant costs and fewer new medicines reaching the market, the impact on the health and well-being of millions of patients could ultimately be devastating as incidences of diseases continue to rise.
Improving the research and development (R&D) productivity of these drug discovery pipelines often requires streamlining the early discovery and development phase to result in fewer attrition rates of candidates that enter the phase of clinical testing and trials. The therapeutic discovery and development process can be divided into 5 stages (Figure 1):
- Target discovery and validation
- Antibody discovery
- Characterization and lead selection
- Engineering and optimization
- Candidate selection and cell line development
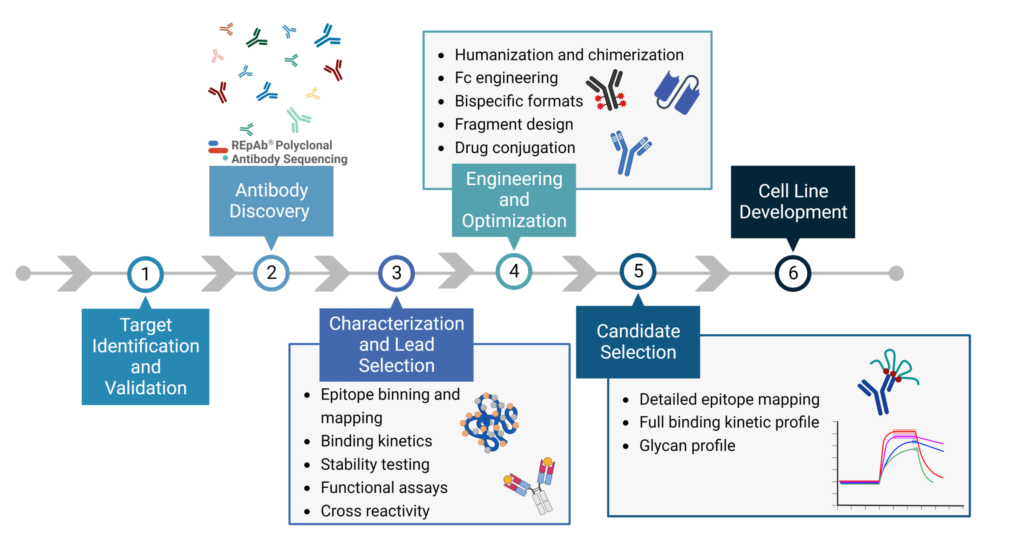
Figure 1. Stages of therapeutic antibody discovery.
Target Discovery and Validation
The wider application of mAb therapeutics requires robust target discovery and validation. Identifying a target for therapeutic intervention requires establishing a correlation between genes/protein products with a diseased state.
Once the target is identified, validation experiments ensure whether the target is a key influence in disease and if its modulation, either through activity or expression produces a desired effect in the population. This is typically done through in vivo and in vitro experiments, such as knockdown models with antisense technology and transgenic mouse models for phenotypic observation. MAbs are common tools for target validation experiments due to their specificity and affinity for their targets, limiting off-target effects.
Antibody Discovery Platform
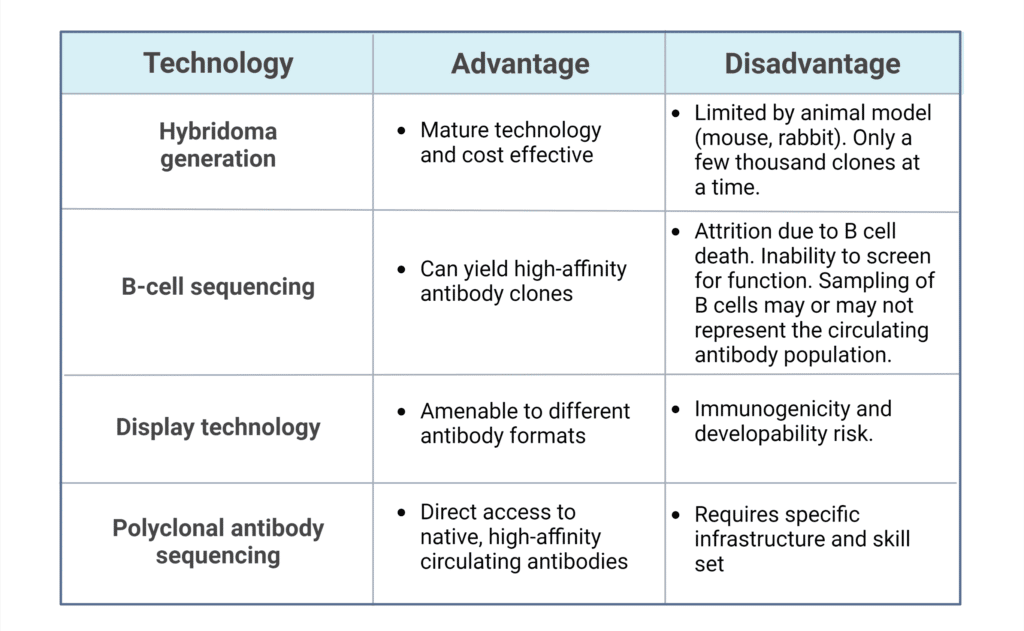
Antibody discovery involves a combination of in vivo and in vitro antibody generation technology. Traditional routes for antibody discovery include: hybridoma generation, B cell sequencing, and display technologies such as phage, bacterial, yeast, or mammalian. Emerging technologies provide additional routes for therapeutic antibody discovery, including de novo polyclonal antibody (pAb) sequencing. Each of these discovery methods have their advantages and disadvantages for therapeutic antibody discovery, summarized in Table 1. To learn more about antibody discovery, refer to our article: Antibody Discovery Platforms.
Table 1: Key advantages and disadvantages of antibody generation technologies for antibody therapeutic discovery.
Polyclonal Antibody Sequencing with REpAb® as a Direct Method for Therapeutic Antibody Sequencing
Polyclonal antibody sequencing with REpAb® is a novel approach to antibody generation pioneered by Rapid Novor (Figure 2). This platform for antibody generation and discovery allows for direct access to high-affinity antibodies from the native immune response, deriving the amino acid sequences of full-length mAbs from a complex polyclonal mixture. The workflow is as follows:
- The serum of an immunized animal with the target antigen is collected.
- The serum is affinity purified to yield an antigen-specific pAb mixture.
- The purified pAb mixture is digested with multiple proteases.
- Digested peptides are analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Using proprietary AI-based bioinformatic algorithms, peptide sequences are assembled de novo to yield full mAb sequences with paired heavy and light chains.
This method of antibody discovery bypasses limitations of other technologies and identifies biologically relevant antibodies from their native source. As such, pAb sequencing is the most direct method for therapeutic antibody discovery. For more information about our REpAb® polyclonal antibody sequencing platform, please refer to our article: What is Polyclonal Sequencing?
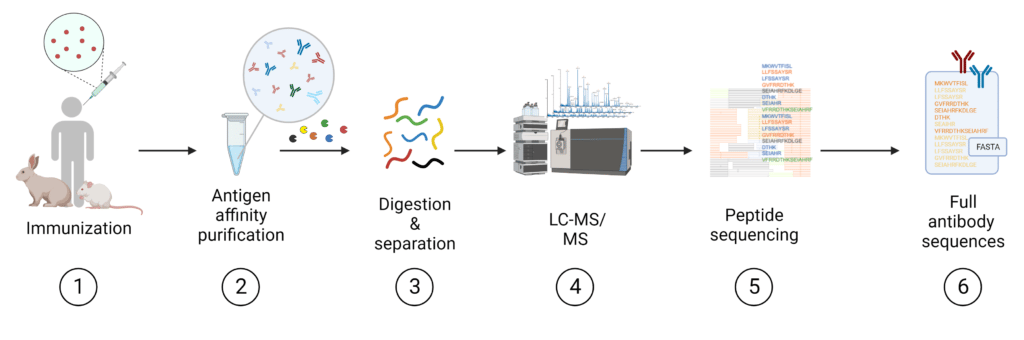
Figure 2. REpAb polyclonal antibody sequencing workflow.
Characterization and Lead Selection
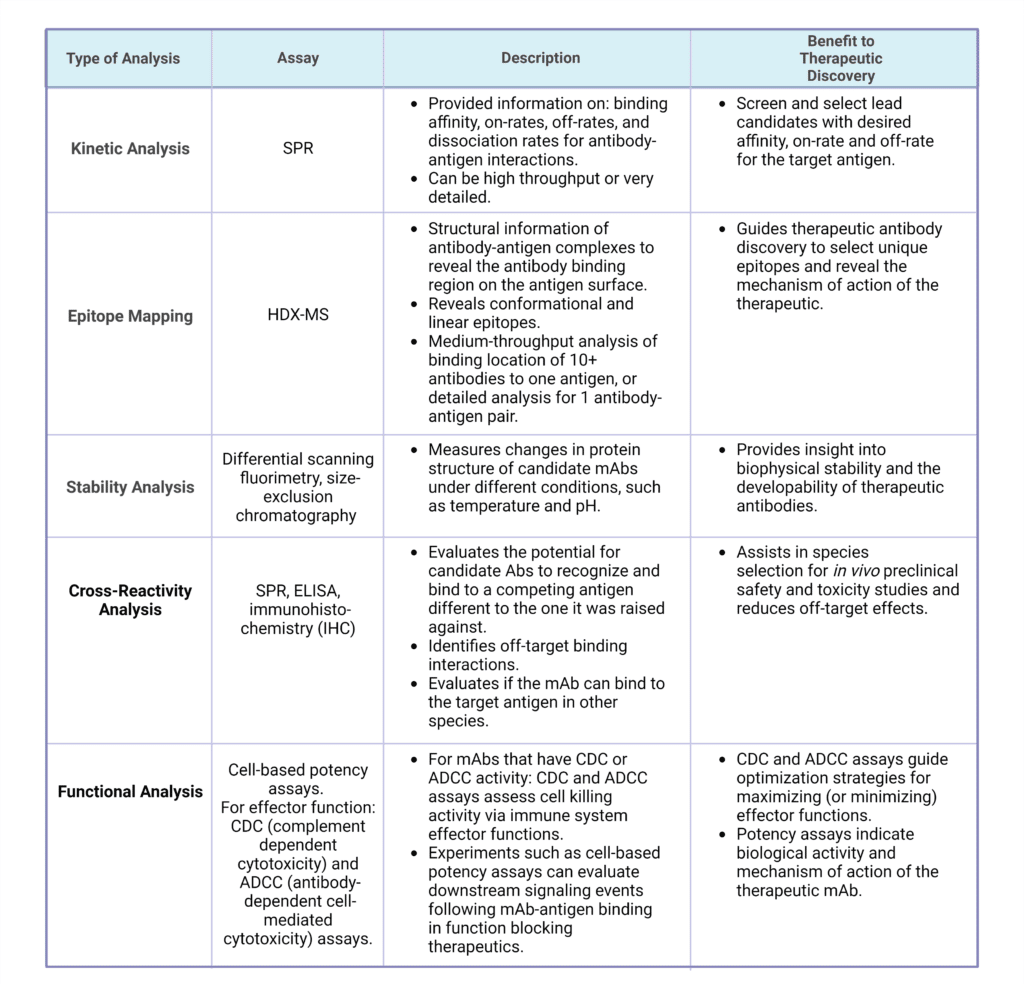
MAbs generated from discovery campaigns are subjected to additional testing and analysis to identify lead candidates with desired properties. Antibody characterization analysis, through technologies such as surface plasmon spectroscopy (SPR) assays, and Hydrogen-Deuterium-eXchange Mass Spectrometry (HDX-MS) can provide information on antibody affinity, specificity, and binding epitopes to select lead mAb candidates. Functional assays, stability testing, and cross reactivity analysis can elucidate additional properties of generated mAbs to help evaluate the therapeutic impact on the immune system. The methods for characterization and functional analysis of antibody therapeutics are described in Table 2.
Table 2: Methods for characterization and functional analysis of antibody therapeutics.
Engineering and Optimization
Antibody engineering and lead optimization uses various techniques and modification strategies to improve the biological properties of mAbs as therapeutic agents. Antibody humanization reduces risk of immunogenicity of the therapeutic mAb by replacing the model species (e.g. murine) peptide sequences with sequences similar to antibody variants naturally produced in humans. Affinity maturation attempts to improve the binding properties of a mAb by altering the sequence. Other optimization strategies include increasing mAb half-life, and reducing or optimizing effector functions through Fc engineering.
Antibody engineering has expanded the use and diversity of mAb therapeutics. Antibodies can be designed to target more than one receptor through efforts in engineering bispecific or multispecific antibodies. Generating antibody derivatives, such as single-chain variable fragments (scFvs) or fragment antigen-binding (Fab) fragments can provide therapeutic advantages such as improved tissue penetration, and alternative drug delivery mechanisms. Additionally, conjugation of chemical payloads for the generation of antibody-drug conjugates provide an additional application of antibody therapeutics.
For more information about antibody engineering, refer to our article: The Hunt for Novel Therapeutics Through Antibody Engineering.
Candidate Selection
After discovery, characterization, and optimization efforts, one or more therapeutic antibody candidates are chosen. The candidate therapeutic mAb should have strong therapeutic potential, with optimized activity and developability. This stage of development involves in-depth characterization and considers cell line development and production strategies for the therapeutic molecule(s). Antibody manufacturing processes may involve cell line development by utilizing mammalian cells as expression systems for mAb therapeutics. Following antibody production, peptide mapping validates antibody candidates and ensures reproducibility during every step of therapeutic development. Engineered and optimized antibodies sometimes behave differently than their originating mAb. As such, in-depth epitope mapping via HDX-MS and full kinetic analysis via SPR can fully characterize the therapeutic candidate. Additional quality assessments are performed following cell-line development, such as glycan analysis, sequence variant analysis, and host cell protein analysis.
Antibody Discovery Services and Characterization with Rapid Novor
Antibodies have become increasingly common in the therapeutic landscape against cancer and various infectious diseases. Rapid Novor supports antibody drug discovery through de novo antibody sequencing and proteomics. Applications can include:
- REpAb® therapeutic antibody discovery service – Exploit the potential of the native immune response
- REmAb® monoclonal antibody sequencing service – Derive sequences from known binders to inform proof of concept studies and mechanism of action studies
- HDX-MS epitope mapping service – Identify linear, conformational, and structural epitopes
- SPR kinetic binding analysis – Measure key antibody properties (ie. binding affinity, specificity, kinetics)
- MATCHmAb™ peptide mapping service – Maintain reproducibility and protect your IP
To learn more about our services, inquire with our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics