 Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: February 10, 2025
Introduction
Successful drug discovery begins with target selection and validation. Improving target validation and conducting early proof-of-concept studies can reduce attrition rates in phase II clinical trials by approximately 24%, ultimately lowering the cost of developing new therapeutics by about 30%.
Antibodies are essential and versatile tools in target identification and validation. They enable the characterization of target distribution, cellular localization, function, and roles in disease contexts. In this article, we’ll explore the process of target identification and validation within the drug development pipeline, as well as strategies to customize antibody tools to enhance their use and application.
Process of Target Identification and Validation
In drug discovery, target identification involves collecting and analyzing data to pinpoint specific biomarkers, proteins, enzymes, or signaling pathways associated with a disease. The next step, target validation, confirms their suitability as potential therapeutic targets with clinical relevance.
Effective target identification and validation require careful evaluation across multiple factors (Figure 1):
- Linkage to Disease Pathology: Demonstrating a direct role of the target in the underlying disease mechanisms and ensuring the availability of relevant model systems. One of the primary reasons new drugs fail to demonstrate efficacy in clinical trials is the lack of robust data showing a causal link between the drug target and the disease, or an insufficient understanding of the target’s role in the underlying disease pathophysiology.
- Target-Related Safety: Addressing potential safety concerns, such as toxic effects resulting from modulating the target’s biological function. Target expression and tissue distribution are also key factors: if a target is highly expressed in non-relevant organs, its functional role in those organs must be carefully assessed to avoid adverse effects.
- Availability and Use of Tool Reagents: Ensuring the availability of tool reagents such as small molecules, peptides, or antibodies. These reagents must be validated, and exhibit high specificity and selectivity with adequate controls to confidently study the target’s relationship to the disease phenotype.
- Strategic Considerations: Evaluating intellectual property (IP) issues and aligning the target with unmet clinical needs and the competitive landscape.
- Feasibility: Evaluating the target’s druggability and assayability to determine whether it can be effectively targeted and studied.
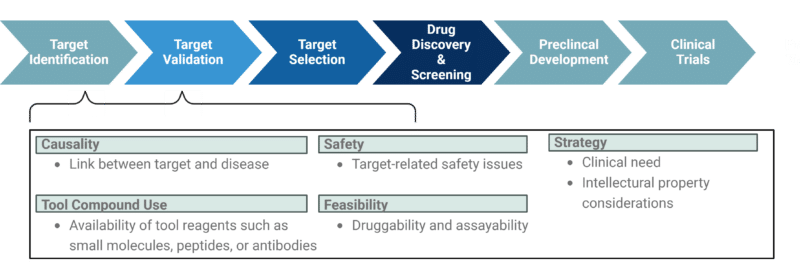
Figure 1. Important aspects of target identification and validation in the drug discovery pipeline.
Antibodies as Tools for Target ID and Validation Experiments
Antibodies play a large role in target identification technologies, enabling the analysis of proteomic changes in disease through applications in biomarker discovery, phenotypic screening, cellular pathway mapping, and structural protein analysis (Figure 2).
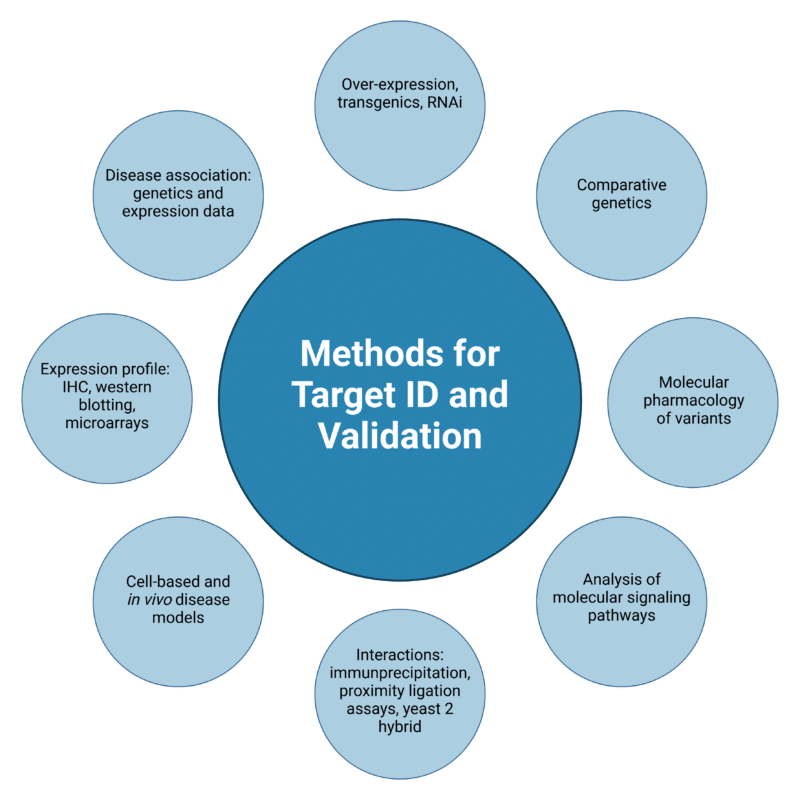
Figure 2. Methods for target identification and validation.
Antibody Microarray for Protein Profiling and Biomarker Identification
Antibody microarrays are a powerful high-throughput tool for profiling protein expression and identifying biomarkers in complex biological samples. In this approach, antibodies are immobilized on a solid surface, allowing target proteins in a sample—such as human serum—to bind to their specific antibody counterparts. Detection is achieved through fluorescent labeling or secondary reagents, revealing changes to protein expression or activity. This technology enables the simultaneous, high-throughput analysis of thousands of protein targets.
Antibody-Based Phenotypic Screening Assays
Antibodies are utilized in phenotypic screening assays, where they help assess or influence protein function in biological processes. Antibody-based phenotypic assays enable the analysis of responses to various stimuli, offering insights into disease mechanisms and treatment pathways. Compared to RNA-based modulation, antibodies may better replicate the effects of small molecules, making them a valuable approach for target identification.
Some antibody-based phenotypic screening assays employ combinatorial antibody libraries delivered via lentiviruses, similar to phage display libraries. These lentiviral libraries can generate up to ∼1.0 × 10⁷ unique antibodies, which are produced and secreted by infected eukaryotic cells containing a fluorescent reporter linked to a target receptor, creating a direct connection between genotype and phenotype. After infection, fluorescent cells are sorted, and the integrated genes encoding agonist antibodies are recovered for further analysis.
One of the major strengths of phenotypic antibody screening is its emphasis on functional activity rather than predefined target specificity. This unbiased approach allows for the discovery of novel drug targets and unexpected mechanisms of action without being limited by existing knowledge of target biology. Furthermore, antibodies identified through this screening process can be directly used for validation studies and, in some cases, developed as therapeutic candidates.
Validating Targets with Immunoassays
Immunoassays are used for validating potential drug targets by assessing their expression, functionality, localization, and interactions within biological systems. High-throughput analyses, such as mass spectrometry or antibody microarrays, provide broad protein profiling but are often followed by targeted immunoassays for in-depth characterization of specific targets.
Techniques like enzyme-linked immunosorbent assays (ELISA), western blotting, and immunohistochemistry (IHC) allow precise quantification and visualization of protein expression and subcellular localization. Additionally, immunoprecipitation and proximity ligation assays (PLA) help investigate protein-protein interactions, revealing key functional insights.
Antibody Applications in Structural Protein Analysis
Antibodies can be used as chaperones for structural biology studies of the potential therapeutic target, where they are used to stabilize proteins for structural determination. By binding to specific epitopes, antibodies can lock proteins into conformations that are suitable for crystallization or cryo-electron microscopy. Typically, antibody formats such as scFvs, Fab fragments, nanobodies, and DARPins can aid in the crystallization of “uncrystallizable” targets. This stabilization is particularly important for membrane proteins and other challenging targets that are often too dynamic for traditional structural techniques.
Custom Antibody Development for Target Validation
The process of target identification and validation relies heavily on access to reliable tool reagents that enable scientists to investigate a target’s role in disease and assess its potential as a therapeutic focus.
High-throughput techniques, such as antibody microarrays offer an approach to target identification by enabling the simultaneous detection of multiple proteins. Theoretically, their throughput is limited only by the number of antibodies incorporated into the array. Unlike DNA microarrays, where probes can be efficiently synthesized at scale, antibodies must be individually produced and extensively validated. The ongoing challenge of obtaining well-characterized antibodies remains a significant hurdle in the field.
Additionally, many targets pose unique challenges, including a limited selection of existing antibodies or highly specific requirements that off-the-shelf solutions cannot meet. Function-specific antibodies, such as chaperones for structural analysis by cryo-EM and X-ray crystallography, are also not readily available.
Strategies for Customizing Antibody Reagents
To overcome the limitations of existing antibodies or to meet specific experimental requirements, several strategies can be employed to customize antibody reagents. These approaches provide researchers with tailored tools to enhance the precision and reliability of target identification and validation, ensuring more robust data and greater flexibility in experimental applications.
- Antibody Engineering: If commercial antibodies lack compatibility with specific experimental applications, antibody engineering can enhance their versatility. Access to the amino acid sequence enables strategies like backbone switching, fluorophore or signal molecule conjugation, and antibody fragment engineering, expanding their use in techniques such as immunofluorescence, ELISAs, and PLA. These sequence-based modifications are unrestricted for research use.
- Polyclonal to Monoclonal Antibody Conversion: In cases where polyclonal antibodies have shown promise but lack the desired consistency and reproducibility, converting them to recombinant monoclonal antibodies can provide a more stable and uniform reagent. This process allows for the identification of specific antibodies that when combined into a mAb cocktail can recapitulate the original pAb while eliminating variability. The result is a more reliable tool for target validation and therapeutic development.
- Antibody Discovery Campaign: One strategy for obtaining antibodies for custom target identification and validation is to initiate an antibody discovery campaign to generate highly specific and tailored antibody reagents. Unlike off-the-shelf antibodies, which may have limitations in specificity, affinity, and application compatibility, a custom discovery process enables precise control over key attributes such as epitope binding, binding strength, and cross-reactivity. However, the cost of generating antibody panels can be prohibitive, often leading researchers to seek catalogue antibodies as an alternative.
Custom Antibody Services with Rapid Novor
High-quality reagents are crucial for robust target validation, but they may not always be available for your specific target or species. Rapid Novor offers mass spectrometry-based services to create and develop customized antibody reagents, streamlining target validation and enhancing the efficiency of proof-of-concept studies.
- REpAb ® polyclonal antibody sequencing can convert tried and true polyclonal antibody reagents to monoclonal cocktails to reap the benefits of pAbs with the reproducibility of mAbs, directly from a purified protein mixture.
- REmAb ® monoclonal antibody sequencing can obtain the amino acid sequence of key monoclonal antibody reagents, even if they have been frozen for years, to facilitate antibody engineering, or reagent reproducibility and sequence immortalization.
- RapidHDX-MS epitope mapping service can be used in target validation, ensuring specificity and efficacy of the antibody reagent to a relevant epitope, and contributing to structural biology studies of the target.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

