Antibody Intellectual Property Protection.
IP Protection for Antibody-Based Assets.
Due to the rapidly changing nature of antibody-based technologies, it is important to co-develop patent strategies alongside any invention. To obtain patent protection, the invention must pass certain requirements including, but not limited to: novelty, inventive step/non-obviousness, and sufficiency.
IP authorities like the USPTO and EPO subject antibody-related patent applications to rigorous scrutiny throughout the prosecution process. Strengthening patent applications often involves providing critical data, including:
- Sequence and structural details
- Identification of target binding epitopes
- Functional analysis through binding kinetics
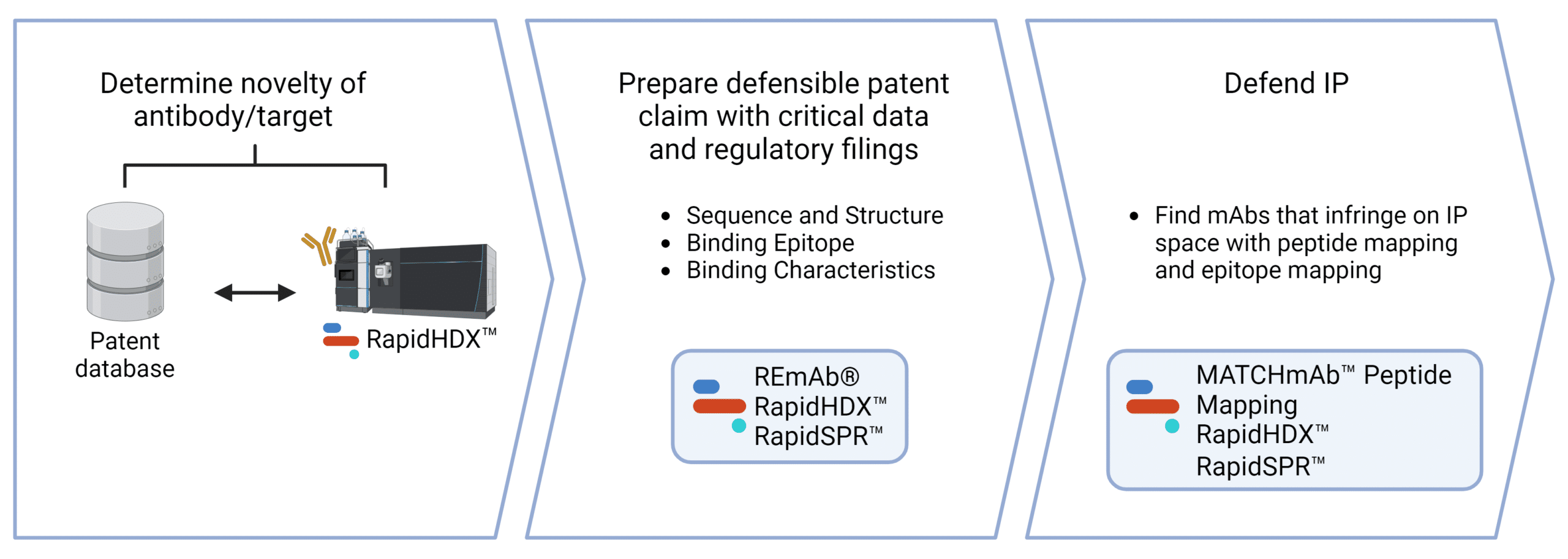
Services for Intellectual Property Protection.
We support the application process in obtaining protection for your IP. Contact us to discover how we can help with patent protection.
De Novo mAb Sequencing
Protect your mAbs and other antibody formats for therapeutic, diagnostic, research applications by obtaining their amino acid sequences and claim their composition of matter for original patent applications.
Explore Monoclonal Antibody Sequencing Services
HDX-MS Epitope Mapping
Characterize and specify specific antigenic epitopes for your antibodies as the scope of protection in patent applications.
Explore HDX-MS Epitope Mapping Services
SPR Binding Kinetics
Strengthen patent applications with functional data by characterizing specific binding properties of your antibodies.
Explore SPR Analysis Service
Peptide Mapping Sequence Confirmation
Strengthen patent applications with functional data by characterizing specific binding properties of your antibodies.
Explore Peptide Mapping Services
Antibody Characterization Services
Our deep expertise working with antibodies, high throughput mass spectrometry and advanced software will give you critical insights in your antibody.
Explore Antibody Characterization Services
Application Publications.
“We’re focused on coupling cytokines with a highly engineered VHVL of an antibody. So by sequencing the antibodies with Rapid Novor we are able to pull out the CDR to graft them into our platform and test in vitro and in vivo.”
Pavel Khrimian – Co-founder and CBO, Deka Biosciences
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics