Published: August 26, 2024
Introduction
The historical use of mice for monoclonal antibody generation has been driven by their robust immunological responses, the efficacy of hybridoma technology, and the capability for genetic manipulation to create humanized models. Despite this, immune responses against highly conserved human proteins are often weak in mice, producing low-affinity or non-specific mAbs. This leaves valuable drug targets and candidate mAbs for novel antigens unexplored.
This article discusses advantages of conducting antibody discovery campaigns in different hosts, and highlights the distinct features of antibodies produced by chickens, camelids, bovine and more.
Advantages of Antibody Discovery in Diverse Species
To overcome the challenges associated with identifying novel antibodies against drug targets, the field is pivoting toward antibody discovery in diverse species, such as camelids, chickens, and bovine. The use of different animal species for antibody discovery offers several benefits, particularly when targeting highly conserved or difficult drug targets.
However, when developing these antibodies for therapeutic use in humans, it is crucial to employ strategies to mitigate immunogenicity, such as antibody humanization.
Tolerance Busting Strategies
Immune tolerance occurs when the immune system becomes unresponsive to specific antigens, often due to repeated exposure. This is especially challenging in antibody discovery against mammalian targets because of high sequence homology between mammalian species.
Immune tolerance can be avoided by using a different species, leveraging the unique immunological responses of phylogenetically distinct animals (Figure 1).
Choosing an antibody discovery host in a species that is phylogenetically divergent from the source of the antigen can mitigate these challenges. Chickens, for example, are frequently used as a non-mammalian host for antibody discovery, and produce a robust immune response against human and mammalian-derived antigens.
This approach not only enhances the variety of potential therapeutic candidates but also addresses the challenges posed by immune tolerance and high sequence homology.
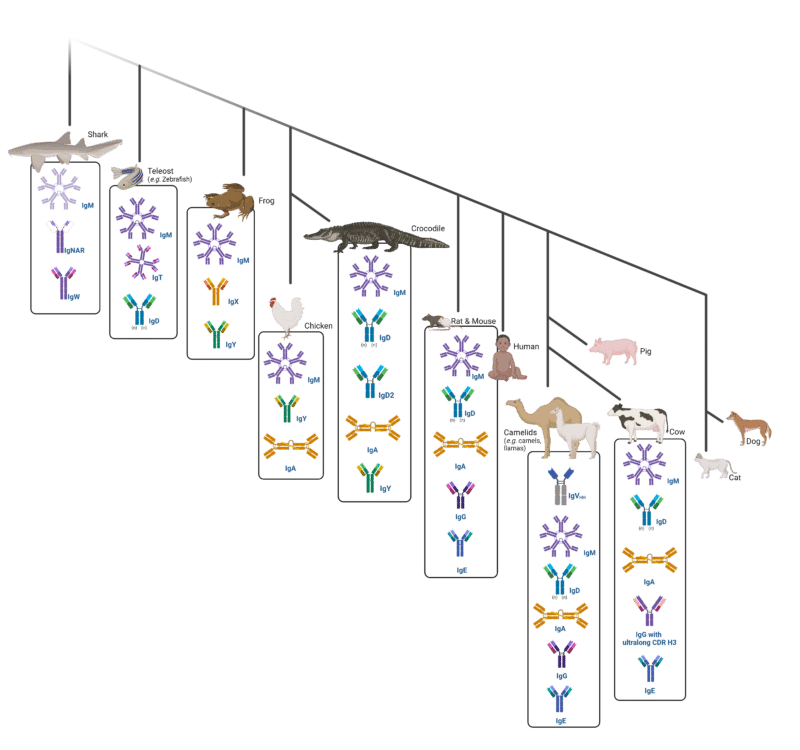
Figure 1. Phylogenetic tree showing the natural evolution of immunoglobulins across many species.
Increased Epitope Diversity
The genetic diversity between species means that each has evolved different sets of B-cell receptors (BCRs), contributing to the recognition of a broader range of antigenic determinants and epitope diversity.
In humans and mice, for instance, the structural diversity of BCR repertoires along the B-cell differentiation axis has been extensively studied. There are species-specific structural predeterminations and dynamics of the complementarity-determining regions (CDRs) of BCRs across different stages of B-cell differentiation.
Other species have unique mechanisms of BCR differentiation, which have the potential to yield diverse antibody responses. Sharks, for example, are one of the oldest living species with adaptive immune systems. They not only express unique antibody formats such as immunoglobulin new antigen receptor (IgNAR), but they also utilize non-canonical mechanisms for BCR and T-cell diversification, which are distinct from those in mice and humans.
Exploring the antibody repertoires of distinct species creates an avenue for unique epitope diversity, as each species’ uniquely evolved immune system responds to antigens differently.
Unique Antibody Formats
Conducting antibody discovery and generation campaigns in species that produce unique antibody formats offers a strategic advantage in targeting hard-to-access epitopes. Species such as camels, and llamas naturally produce antibodies with distinct structural features, such as heavy chain only antibodies (HCAbs).
The complementarity determining regions (CDRs) of antibodies are crucial for antigen interactions. Of the six total CDR regions in immunoglobulins (three on the heavy chain and three on the light chain), HCDR3 (the third CDR region on the heavy chain) is the most important for antigen binding in mammals. The length of the HCDR3 varies between different species. Bovines produce antibodies with ultralong CDR3s that form a unique protruding “stalk and knob” structure extending from the antibody’s surface. This structure has the potential to access functionally important and hidden epitopes.
By harnessing these species-specific antibody formats, researchers can expand the repertoire of potential therapeutic candidates and enhance the likelihood of discovering antibodies that target novel epitopes with unique functionality.
Mitigating Cross-Reactivity
Antibodies generated in divergent species are less likely to cross-react with human immunoglobulins and other self-antigens, thereby improving their specificity and reducing the potential for off-target effects.
In addition to therapeutic applications, antibodies from divergent species can be used in diagnostic tools with reduced background noise and increased signal clarity. For instance, in immunoassays, antibodies from species like chickens or sharks can detect human antigens with minimal interference from human immunoglobulins, leading to more accurate and reliable diagnostic results.
Unique Features of Antibodies from Diverse Hosts
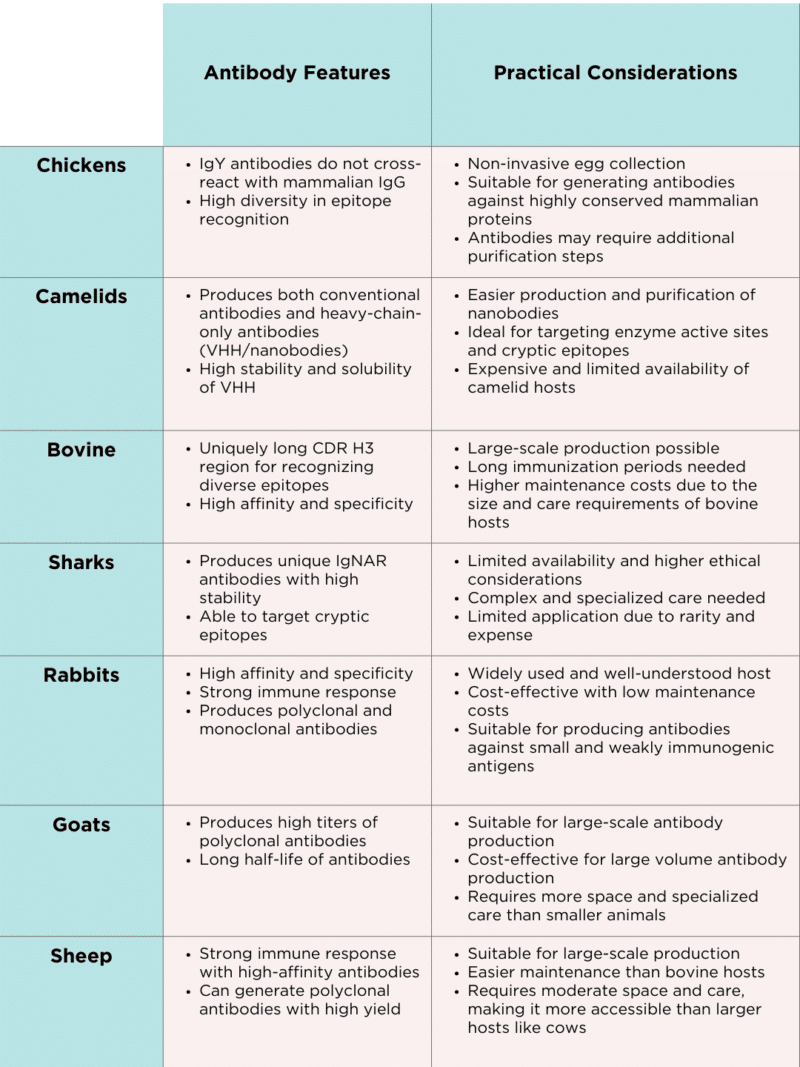
Exploring the unique antibody features across various species reveals the unique diversity in immune responses and antibody formats, with each species offering distinct advantages for research and therapeutic applications. The phylogenetic relationships among these animals highlight their evolutionary paths and how these have influenced the specialization of their immune systems (Figure 1). The key antibody features and practical considerations for different antibody discovery hosts is summarized in Table 1.
Table 1. Antibody features and practical considerations for different antibody discovery hosts.
Chicken IgY Antibodies
Chickens produce an antibody isotype called IgY, which is considered an evolutionary precursor of IgG and IgE in mammals, and is functionally equivalent to IgG (Figure 2). However, there are some features of IgY that differentiate it from IgG: IgY is 180 kDa compared to IgG being around 150kDa (Figure 3). While both are composed of 2 heavy chains and 2 light chains, IgY has four immunoglobulin domains in the constant region of the heavy chain while IgG only has 3. Additionally, IgY lacks a hinge region, limiting its flexibility, but also makes it resistant to proteolytic degradation.
Additionally, as mentioned earlier, their phylogenetic distance from mammals also minimizes cross-reactivity with mammalian tissues, making them ideal for diagnostic assays and therapeutics. Unlike mammalian antibodies (IgG), chicken IgY antibodies are derived from the yolk sac rather than the bloodstream, offering a non-invasive and sustainable source of antibodies.
Chicken antibodies are increasingly utilized in diverse fields such as infectious disease research, environmental monitoring, and even food safety, highlighting their versatility and promising potential in advancing biotechnological and clinical applications.
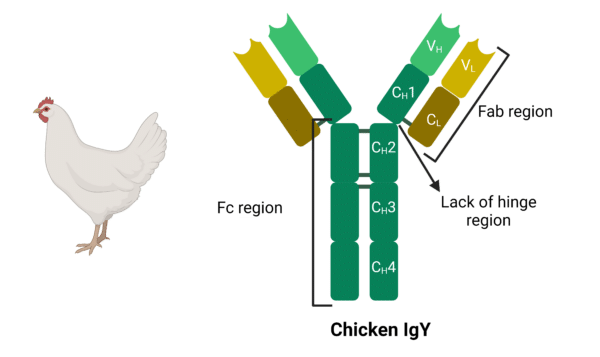
Figure 2. Chicken IgY antibodies are 180 kDa, containing a CH4 domain and lacking a hinge region.
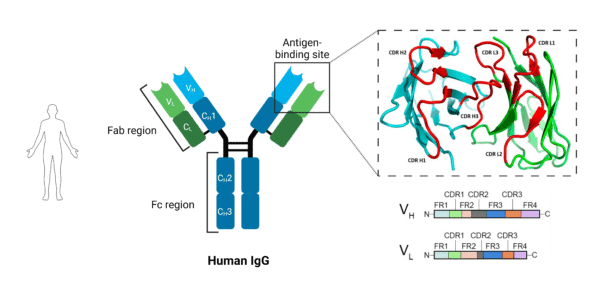
Figure 3. Canonical form of human IgG antibody.
Camelid Antibodies
The camelid family, including camels, alpacas, and llamas produce a unique heavy chain only antibody (HCAbs), made up of only two heavy chains with the variable region alone (VHH domain) (Figure 4). The VHH domain is connected to the hinge region, serving as the single target-binding site that determines the specificity of the antibody. Camelid antibodies are significantly smaller in size than canonical heavy + light chain Igs, due to the lack of light chains and CH1 domains. CHAbs have been engineered to an even smaller format, known as a nanobody, containing only the VHH domain.
There are many attributes of camelids that make them attractive hosts for antibody discovery and generation. Small but powerful, nanobodies are capable of binding to epitopes that are inaccessible to traditional epitopes, further enhanced by their elongated CDR3 loops. Additionally, their small size enhances tissue penetration, reaching distal sites and tumour locations with ease compared to canonical mAbs. They are resistant to high acidity and high temperature and less prone to aggregation, all of which are ideal qualities for developing antibody-based therapeutics.
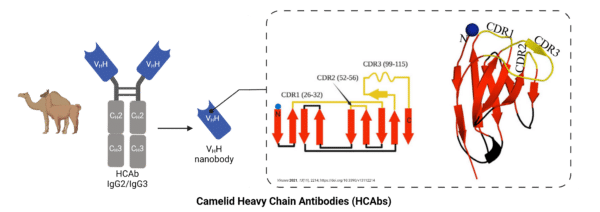
Figure 4. Heavy chain antibody (HCAb) derived from camelid species, including alpacas, llamas, and camels. These antibodies lack a light chain, have an elongated CDR3 loop, and can be engineered into a VHH nanobody.
Bovine Antibodies
Most CDR regions of IgGs contain 6-20 amino acid stretches which contribute to antigen recognition and binding. However, bovines are unique in that they produce IgGs with ultralong CDR regions, extending from 50 to 70 amino acids (Figure 5). These ultralong CDR loops form a “stalk and knob” structure rich in disulfide bonds, and protrude from the surface of the Ig and enhance both antigen binding and antibody stability.
CDR grafting allows the incorporation of ultralong bovine CDR3s into human antibodies. Additionally, bovine CDR3 can be engineered into an independent CDR H3 peptide knob, becoming the smallest antigen-binding fragment that retains the binding properties of the original full-length mAb.
Transchromosomic bovines, which express a human artificial chromosome containing the complete germline sequences of human immunoglobulin heavy-chain and kappa-chain, produce antigen-specific, fully human IgGs. These antibodies are more potent and protective than conventional human antibodies and are produced in much larger quantities. Additionally, hyperimmunization of bovine generates robust immune responses and high yields of pAbs in serum, making them valuable hosts for antibody discovery and generation.
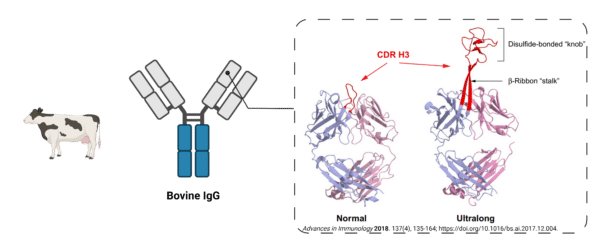
Figure 5. Bovine antibodies follow the canonical antibody format of mammals. Unique to bovine antibodies is their ultralong CDR3 loop, which creates a “stalk and knob” structure protruding from the surface of the antibody.
Shark Antibodies
Cartilaginous fish, particularly those from species like the nurse shark and the elephant shark, exhibit unique features that set their antibodies apart from conventional mammalian antibodies. Notably, shark antibodies include heavy-chain-only antibodies (HCAbs) known as immunoglobulin new antigen receptors (IgNARs) (Figure 6). The variable region of IgNAR, referred to as VNAR, determines the antibody’s specificity.
VNAR subtypes are defined by their cysteine residues, CDR3 lengths, and amino acid variability. Lacking a CDR2 domain, these antibodies depend more on CDR1 and CDR3 to confer binding specificity and affinity, with CDR3 lengths extending beyond 100 amino acids. This extended structure allows access to hidden epitopes on target antigens.
IgNARs are also known for their high chemical and thermal stability, a trait influenced by their evolutionary environment. Their resistance to chemical denaturants and low pH environments makes them particularly attractive for the development of therapeutic drugs, molecular imaging, and diagnostics. The small size of VNARs also allows for diverse methods of drug administration, such as aerosolization, further enhancing their potential in various medical and research applications.
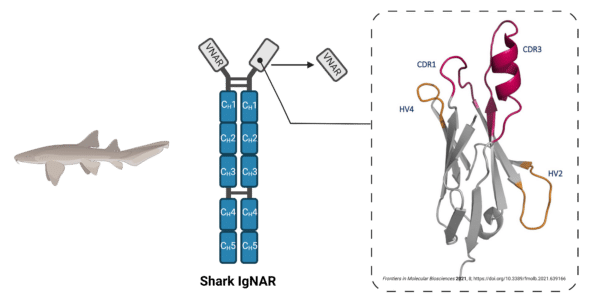
Figure 6. Sharks produce a unique IgNAR antibody format, which is a heavy chain antibody (HCAb) that contains five constant regions in the Fc portion. The VNAR portion, responsible for antigen binding, lacks a CDR2 domain and can be engineered into a single VNAR binding fragment.
Rabbit Antibodies
Rabbits are a significant source of antibodies with broad utility. Being phylogenetically distinct from rodents, rabbit antibodies can recognize epitopes on human antigens that are not immunogenic in mice or rats. The rabbit immune system is especially advantageous for generating antibodies against small molecules, peptides, and post-translational modification sites, which might be non-immunogenic in mice.
Moreover, rabbit antibodies tend to undergo a higher affinity maturation process, resulting in antibodies with tighter binding to their targets. They offer increased sensitivity without loss of specificity compared to mouse-derived antibodies, often displaying affinities 10-100 times higher. These characteristics make rabbit antibodies excellent candidates for use in diagnostic assays, where high sensitivity and specificity are critical.
This distinction between rabbits and rodents contributes to increased epitope diversity and enables the generation of antibodies that cross-react with mouse orthologs of human antigens. This capability is crucial for preclinical research using human tumor xenograft models to determine off-target and on-target effects of therapeutic antibodies.
Antibody Discovery Platforms for Diverse Hosts
Antibody discovery platforms have evolved to leverage diverse hosts such as rabbits, llamas, and sharks, each offering unique advantages in generating a wide variety of antibodies. These platforms utilize advanced techniques such as phage display, yeast display, and next-generation sequencing to explore the antibody repertoires of these hosts. However, despite their potential, there are limitations in existing antibody libraries and genomic information that can hinder the discovery of novel antibodies.
One significant challenge is that these libraries do not fully capture the natural variability of the host’s immune system. Additionally, the genomic information for some hosts (especially non-traditional ones like sharks and llamas) is often incomplete or poorly annotated, complicating the identification and characterization of novel antibodies. These limitations can result in missed opportunities for discovering antibodies with unique functionalities.
Species Agnostic Antibody Discovery Platform
To overcome the limitations in discovering the complete and functional immune repertoire of diverse antibody generation hosts, a species-agnostic antibody discovery platform is crucial.
Rapid Novor’s REpAb® antibody discovery service uses de novo polyclonal sequencing to directly sequence antibodies in serum using mass spectrometry, thereby bypassing the need for genomic information. Even when genomic information and IgSeq data is available, polyclonal sequencing from serum can identify antibodies not found in B cell sequencing.
For more information on de novo polyclonal sequencing for antibody discovery, check out our article: What is Polyclonal Antibody Sequencing?
REpAb antibody discovery has been conducted in over 9 species, including alpaca, bovines, chickens, dogs, goats, sheep, and humans. Our previous case studies have highlighted the application of polyclonal sequencing for diverse hosts:
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics