August 4, 2021
What is Antibody Validation?
Antibodies are used in a variety of ways in academia and industry, from tools to therapeutics. Because antibodies are produced using live processes, which are naturally error-prone, validation is required from time to time (Lamanna et al. 2017). Furthermore, to develop biological therapeutics, the protein sequence must be confirmed as part of the regulatory process (Scott et al. 2014).
Why Do Antibodies Need Validation?
Antibody discovery systems are biological in nature, and are thus not immune to errors. Hybridomas, for instance, throughout time, may acquire aberrations that result in additional production of chains, or an antibody with a sequence that has deviated from the original sequence. Furthermore, sometimes hybridomas are not perfectly monoclonal by nature, and may in fact produce more than one antibody.
In the case of single B-cell sequencing, recombinant production is not exempt from eventually encountering problems down the road, as aberrations have been noted in the literature after several passages of cell lines. Phage, yeast and bacterial display carry their own issues, which other than potential DNA errors, include a lack of biologically relevant post-translational modifications (PTMs).
Antibodies are widely used in labs and therefore are quickly consumed; multiple lots from the “same” antibody will be used throughout a year at any given lab. Because lot-to-lot variability is a common issue, it is critical to validate antibodies generated in-house, and those bought commercially to ensure reproducibility of the research or treatment.
How are Antibodies Validated?
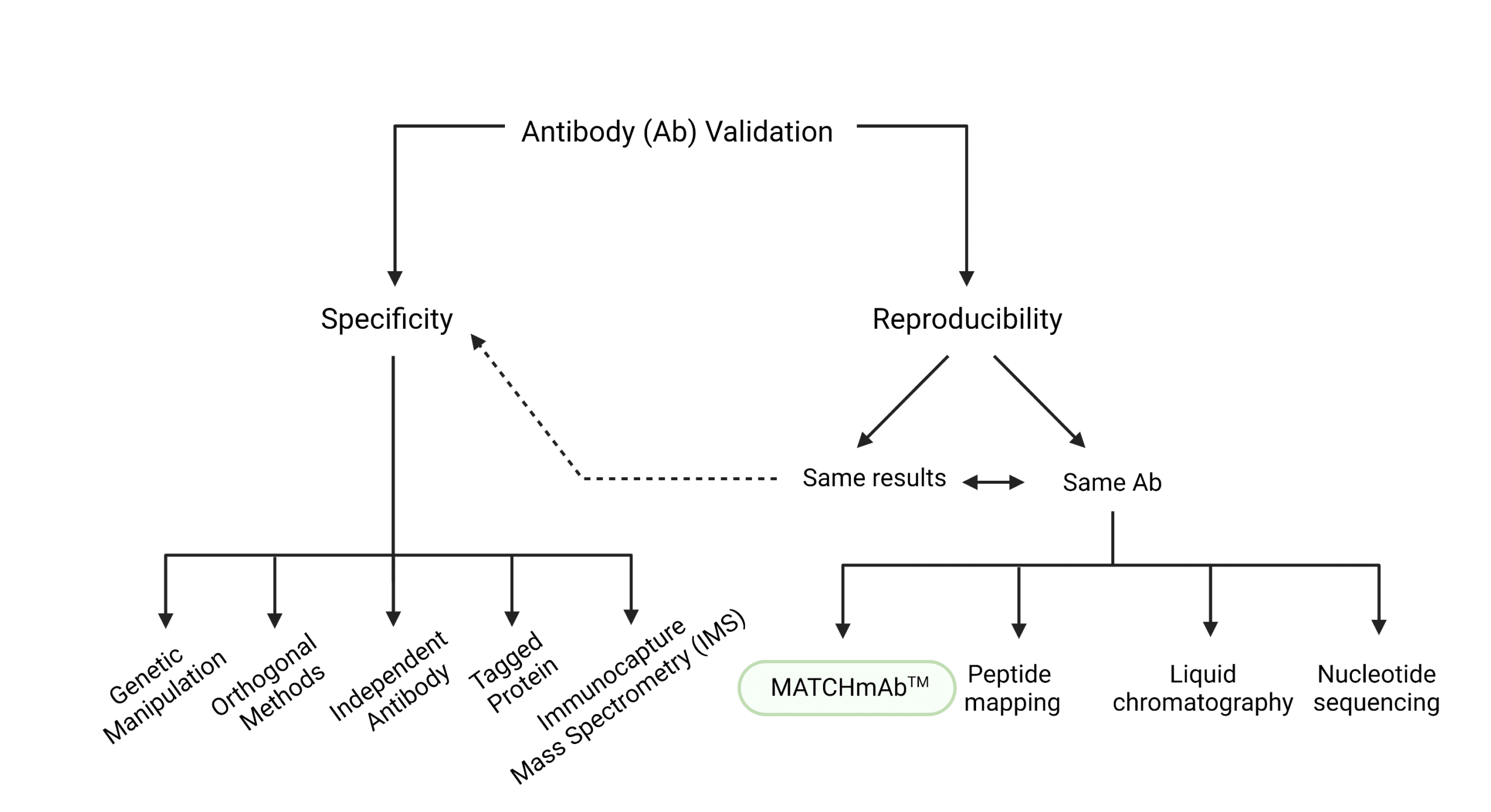
We can think of antibody validation as the authentication of an antibody that certifies its specificity and reproducibility. Specificity refers to an antibody’s ability to recognize and preferentially target a particular protein, and its affinity or strength of binding to that protein. On the other hand, for an antibody to be reproducible, it must give the same results each time, or its protein sequence must remain unchanged.
- Knock-out or overexpression cell lines can validate that the antibody is specific and sensitive to the antigen of interest. Fluorescence should be abolished when antigen is knocked down and should be increased when antigen is overexpressed.
- Immunoprecipitation can validate that the antibody is binding to the antigen of interest. If the antigen is present on the subsequent western blot, then the antibody is specific for the antigen.
- Isotype controls can be used that match the antibody matches the species, subclass, and conjugation of the selected antibody to distinguish non-specific fluorescence.
- Surface plasmon resonance (SPR) can be used to determine the full binding kinetic profile for the antibody-antigen interaction. This technique can tell you precise information about the dissociation constant and affinity of your antibody to your antigen of interest.
- Immunohistochemistry (IHC) and immunofluorescence (IF) can be used to validate antibody performance on tissue sections to confirm specificity and localization of the antigen in situ.
- Treatment of antibody with blocking peptide that corresponds to the antigen sequence can be used to confirm the specificity of the antibody. If the fluorescence is abolished after treating the antibody with the blocking peptide, then the antibody is specific to the antigen of interest.
- Positive and negative controls that are known to either contain or not contain the antigen of interest can be used to validate the antibody specifically and set the level of background staining.
Alternative techniques such as enzyme-linked immunosorbent assay (ELISA) and western blotting can also be used to validate antibody specificity.
Antibody Validation Testing for Specificity
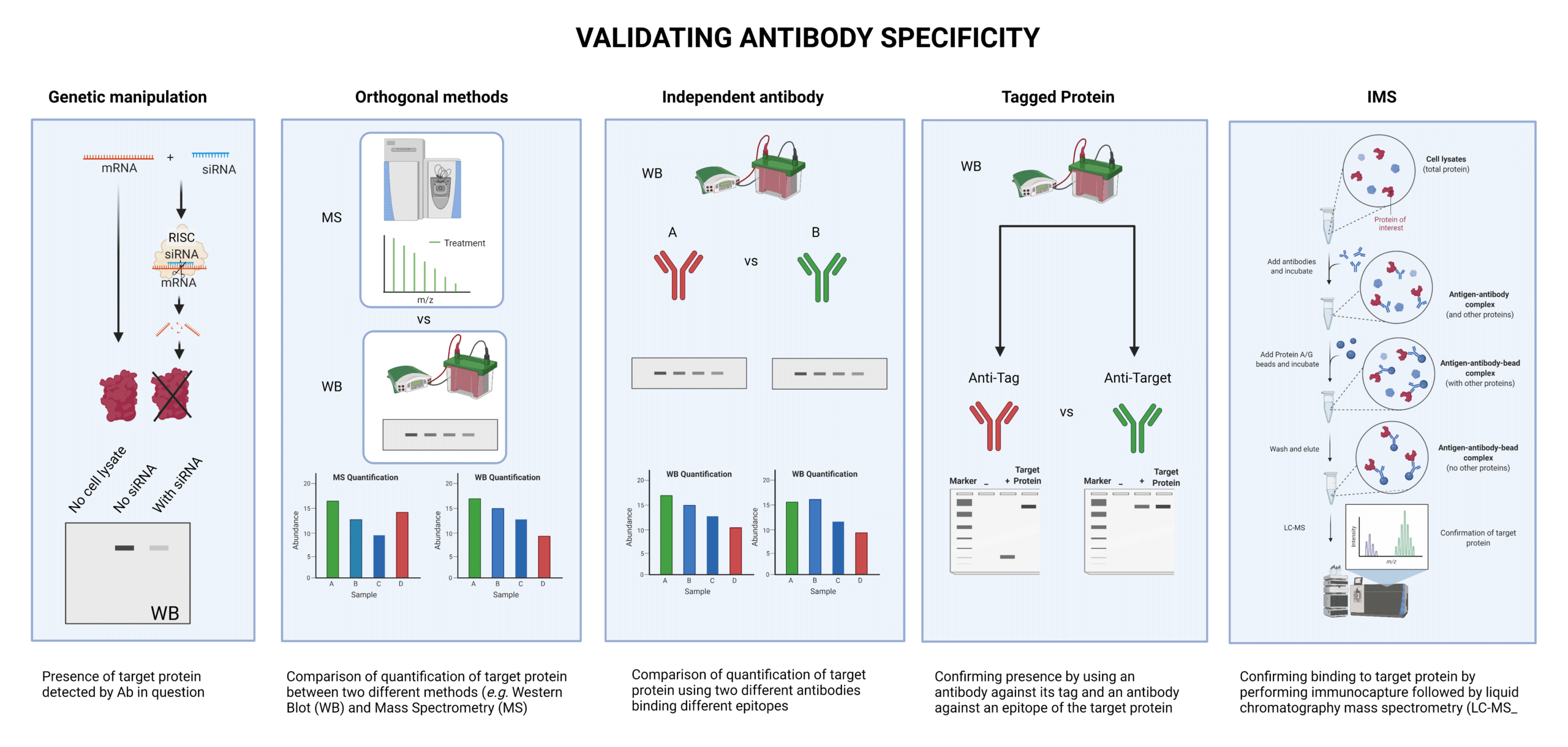
Specificity can be tested in a variety of ways:
- Using siRNA to knockdown the gene of interest and drastically reduce the expression of the target protein (genetic manipulation),
- Comparing quantification of the target protein with the same antibody by two different methods, such as Western blot (WB) and mass spectrometry (MS) (orthogonal methods)
- Confirming for the presence of the protein, -With the antibody being tested and an independent antibody that binds a different epitope (independent antibody) -With an antibody that binds the tag attached to the protein, and an antibody that binds an epitope in the target protein (tagged protein)
- Performing immunocapture MS (IMS), which is the process of binding the antibody to the target, performing immunoprecipitation. and running the output on MS to detect the target protein.
Since research studies conduct experiments that allude to the specificity of a commercial antibody, academic and industry scientists often also rely on publication references to decide which commercial antibody to purchase. We have compiled databases that store such information here.
Antibody Validation Testing for Reproducibility
Antibody reproducibility is often tested by:
- checking that physical binding occurs, either through surface plasmon resonance, immunoprecipitation, or specificity-minded testing methods, or,
- checking that the sequence remains the same by performing MATCHmAb™, traditional peptide mapping, liquid chromatography, or nucleotide sequencing.
MATCHmAb™ peptide mapping service is a sequencing service researchers can use instead of traditional peptide mapping to have a quick and affordable way of verifying the protein sequence . The sequence obtained through MATCHmAb™ can be used to:
- meet regulatory guidelines,
- confirm the sequence of a biosimilar, or
- validate the sequence of an in-house antibody.
MATCHmAb™ data can be used to satisfy sequence confirmation that is often required during the regulatory process for biological drug development (Scott et al. 2014). Confirmation of the original sequence ensures a higher likelihood that the antibody will behave the same. An in-house specificity-minded experiment, such as an ELISA or WB can be performed in parallel to MATCHmAb™ for a simpler yet comprehensive orthogonal approach.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics