Written by:
Yuning Wang, PhD
Updated: January 18, 2023
(Published: January 21, 2022)
Discovery of Camelid Antibodies
Three decades ago, the discovery of a peculiar type of antibody from camel sera forever changed people’s conventional knowledge of immunoglobulin structure. The dogma at the time was that all naturally-occurring antibodies consisted of two heavy chains and two light chains; this structure was thought to be essential for antigen recognition. However, curiously, the unearthed camel antibody was devoid of light chains and still fully capable of antigen-binding.
What are Camelid Antibodies?
Camelid antibodies are antibody isotypes called heavy chain-only antibodies (HCAbs). Heavy chain-only antibodies are found across all members of the Camelidae family including camels, llamas, and alpacas (Figure 1 highlighted box, red arrow); a homologous version of camelid antibodies is also found in cartilaginous fish such as sharks (Figure 1 top left box).
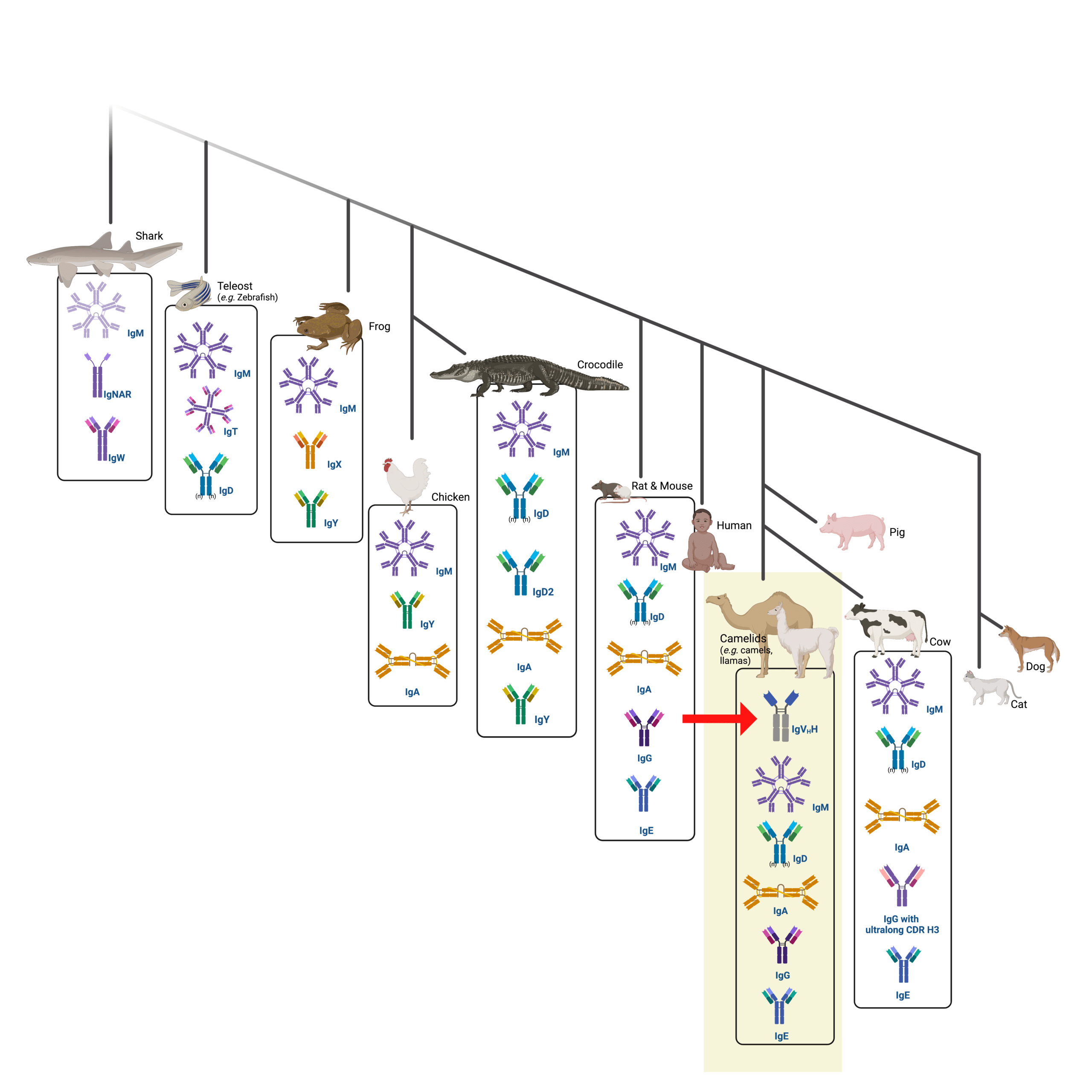
Figure 1. Infographic of a phylogenetic tree showing the natural evolution of immunoglobulins across many species, of which the camelid family is highlighted in yellow. The red arrow points to one of their expressed antibodies: the single-chain antibody (HCAb).
Structure of Camelid Antibodies and Nanobodies
A canonical antibody consists of two heavy chains and two light chains, both of which contribute to two identical antigen-binding sites. In contrast, camelid antibodies are made up of only two heavy chains with the variable region alone (without CH1), referred to as the VHH domain. The VHH domain is directly connected to the hinge region, serving as the single target-binding site that determines the specificity of the antibody. The absence of light chains and CH1 domains results in camelid antibodies being significantly smaller in size than their other immunoglobulin counterparts (Figure 2).
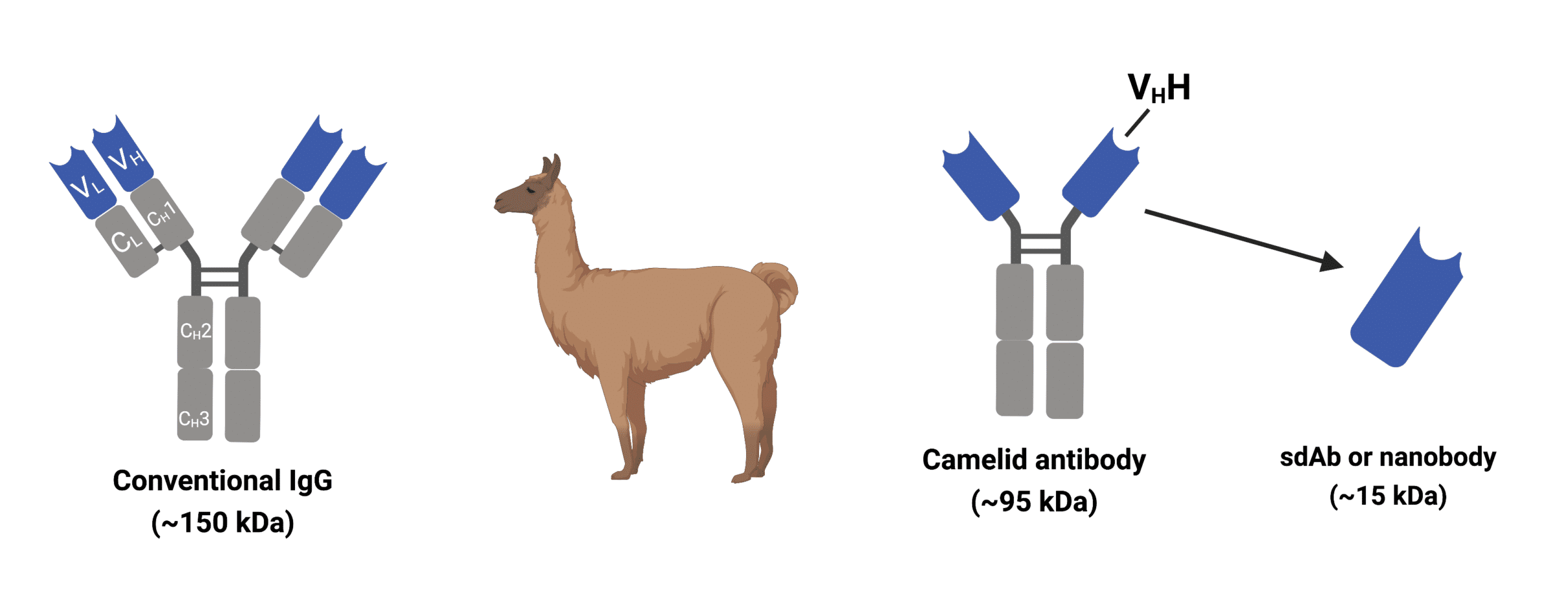
Figure 2. Schematic representation of the camelid antibody and nanobody in comparison to a conventional IgG antibody
Advantages of Camelid Antibodies and Nanobodies
The VHH domains of camelid heavy chain-only antibodies can be effectively engineered, and are designated as single-domain antibodies (sdAbs), or nanobodies, in reference to their small nanometre size range (Figure 2). In addition to many sought-after properties, nanobodies are capable of targeting epitopes of antigens that are inaccessible for conventional antibodies due to their characteristically lengthier CDR3 loops (Table 1).
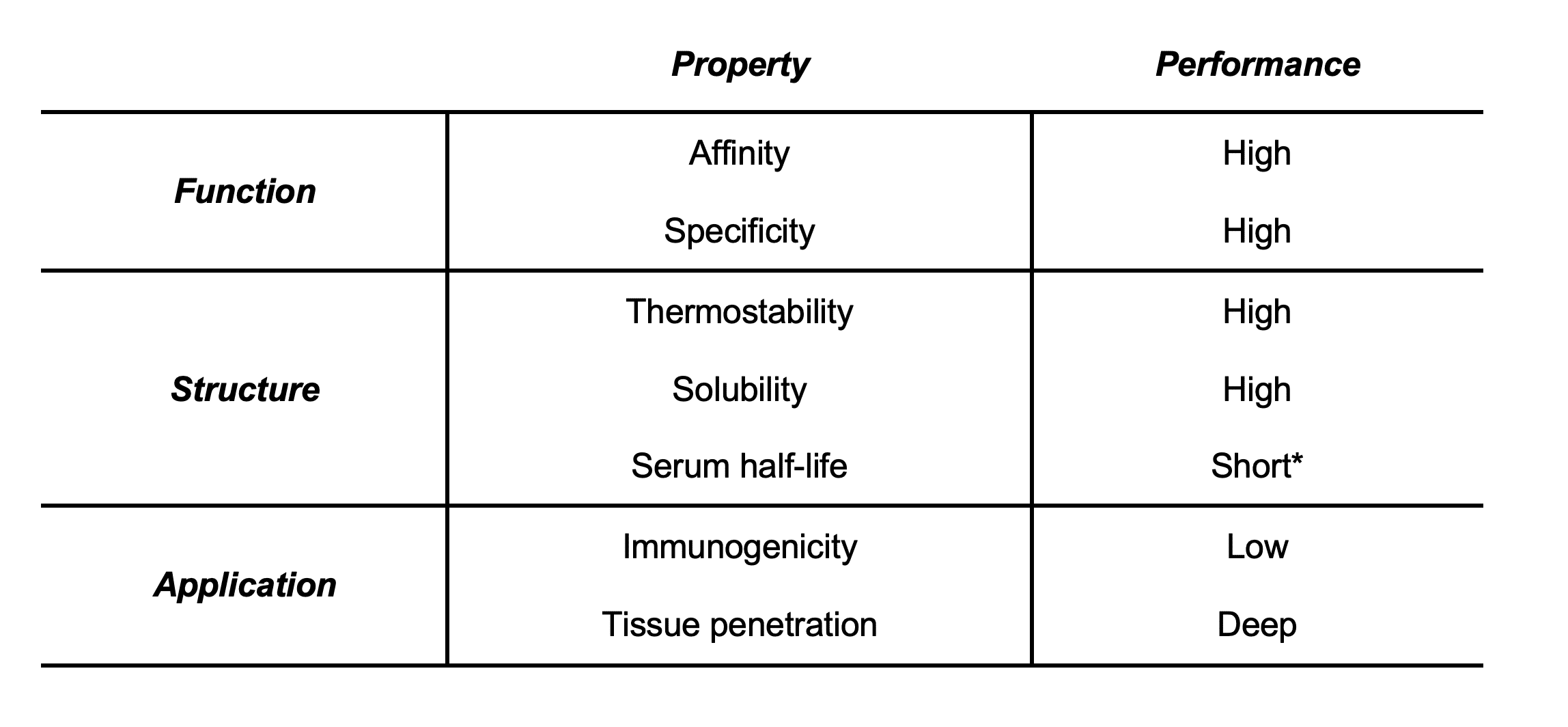
Table 1. Important Properties of Camelid Antibodies. (*Beneficial for certain applications that favour rapid clearance, but not for treatment of viral diseases. In this case, camelid antibodies would require to be readministered multiple times compared to canonical antibodies4.)
Scientists working on antibody therapeutics and diagnostics are also drawn to use nanobodies because of the relative ease at which they can be produced and engineered. Unlike many other antibody fragments such as Fab or single-chain Fv (scFv), recombinant production of nanobodies does not require the association of VH and VL domains, thus avoiding mispairing issues. In addition, the single-domain nature of nanobodies greatly facilitates their genetic engineering into formats suited for a variety of purposes.
Camelid Antibodies and Nanobodies for Therapeutic and Research Applications
Thanks to their advantageous biophysical features, nanobodies have opened new research perspectives in the development of innovative human therapeutics. In February 2019, the FDA approved the first VHH-based nanobody drug, caplacizumab, for the treatment of acquired thrombotic thrombocytopenic purpura (TTP). Caplacizumab was constructed by linking two humanized VHH domains that were initially identified from llamas. Nowadays, hundreds of nanobody therapeutics are under development against various diseases including hematology, infectious diseases, inflammation, and COVID-19, with many being studied in clinical trials.
Benefits of using nanobodies as therapeutics include the following:
- Small but powerful: Target epitopes with high affinity at locations that are inaccessible for traditional antibodies
- Better stability and solubility: Resistant to high acidity and high temperature; less prone to aggregation
- Higher tissue penetration: enter tissues and tumors more rapidly and deeply than traditional antibody drugs allowing for increased efficacy
- Ease of engineering & manufacturing: Flexible to be tailored into different formats facilitating lead optimization; relatively low production cost
For many of the above reasons, nanobodies are also pursued for diagnostic purposes, and for manufacturing reagents to aid in research and development.
How are Camelid Antibodies and Nanobodies Developed?
Prior to producing nanobodies, camelids (typically, llamas) are first immunized with a target antigen. Then, nanobodies are commonly discovered through a nucleotide sequencing approach called next generation sequencing (NGS). NGS is performed on the nucleic material extracted from the llamas’ circulating peripheral blood lymphocytes. Then the heavy chain genes are cloned into a phagemid vector to generate a phage display library, which will be used to perform affinity maturation in vitro. To bypass cell collection and potential contamination, another method can be used to access the circulating nanobodies directly. This method is called next generation protein sequencing (NGPS).
Discovering Camelid Antibodies and Nanobodies through NGPS
NGPS is a robust and complementary technology that can be used to power camelid biologics pipeline from discovery through engineering, development, validation, and manufacturing (Figure 3).
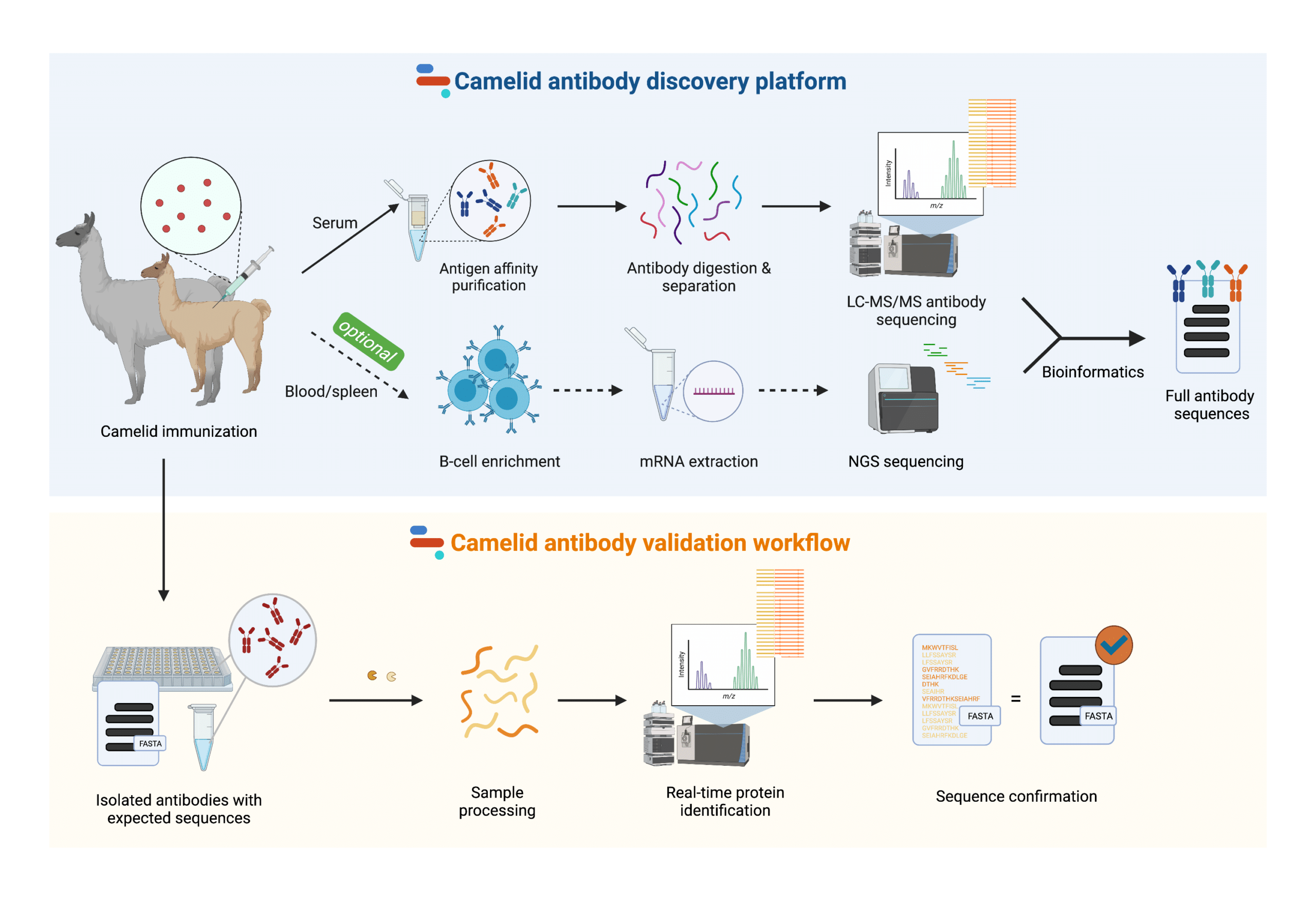
Figure 3. Rapid Novor’s next generation protein sequencing platform for the discovery and validation of camelid antibodies and nanobodies
REpAb® is Rapid Novor’s antibody discovery platform that is rooted in mass spectrometry and supported by big data-powered machine learning. REpAb® can be easily integrated into already existing antibody discovery pipelines utilizing nucleic data, or be directly employed on affinity-purified bleeds from inoculated animals. REpAb® captures the circulating antibody pool already primed against the target and does not require the culling of the production animal. Full sequences of functional antibodies have been directly derived from rabbit, goat, and now alpaca using our REpAb® technology. For more information about our REpAb® antibody discovery platform, please refer to our article: What is Polyclonal Antibody Sequencing?
De Novo Sequencing for Nanobodies
When developing therapeutic antibody fragments and nanobodies, the selection and screening process that usually relies on hybridoma and phage display can be tedious and time-consuming due to the large number of choices for fragment combinations. These processes also do not include relevant information on post-translational modifications (PTMs). By obtaining information on PTM type and location, NGPS can predict potential enhancement or detrimental effects of physicochemical properties, and function of antibodies. As such, NGPS is a powerful tool that can complement and optimize pipelines relying on hybridoma and/or phage display.
Rapid Novor’s antibody discovery service and antibody sequencing services can be used to sequence nanobodies from camelids and enable downstream characterization and engineering. Furthermore, our MATCHmAb™ peptide mapping service offers a quick alternative to traditional peptide mapping of verifying the protein sequence of isolated antibodies using our proven protein sequencing technology to help researchers avoid cross-reactivity and reproducibility problems down the road.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics


