Kierkels, G. J. J., van Diest, E., Hernández-López, P., Scheper, W., de Bruin, A. C. M., Frijlink, E., Aarts-Riemens, T., van Dooremalen, S. F. J., Beringer, D. X., Oostvogels, R., Kramer, L., Straetemans, T., Ucker, W., Sebestyén, Z., Kuball, J. Characterization and Modulation of Anti-αβTCR Antibodies and Their Respective Binding Sites at the βTCR Chain to Enrich Engineered T Cells. Mol Ther Methods Clin Dev 22, 388–400 (2021). https://doi.org/10.1016/j.omtm.2021.06.011.
Abstract
With the approval of the first engineered T cells expressing chimeric antigen receptors (CARs), a next wave of cell therapy interventions will come with T cell receptor (TCR)-engineered T cells.
αβTCR-engineered T cells have been applied in clinical trials, specifically directed against cancer/testis antigens. Though the clinical outcomes are promising, only a small proportion of patients benefit from these novel treatments. Lower response rates are partially attributed to a heterogeneous mixture of non-engineered and poorly engineered T cells that remain in the administered therapeutic product. For successful translation of these novel treatments into the clinic, engineering efforts should be reinforced with effective methods for engineered T cell purification and engineered T cell elimination post infusion into patients.
Researchers from the Kuball Lab at the Center for Translational Immunology, Utrecht University exploited a strategy in which an anti-human αβTCR monoclonal antibody that is routinely used to purify T cells expressing γδTCRs, was the basis for a purification procedure to enrich for αβTCR-engineered T cells.
In this case study, a commercial anti-human αβTCR antibody was found to bind an epitope on the TCRβ chain of endogenous human αβT cells. Further characterization demonstrated that this binding was lost, upon murinizing at least two amino acid residues in the sequence of domain 3 of the human TCRβ chain.
For the depletion of the murinized variant of the αβTCR, researchers looked to identify an antibody that was specific for these variant αβTCRs. A commercially available anti-murine TCRβ chain antibody, of Armenian hamster origin, was assessed for binding and subsequently humanized. In doing so, chimeric variants of the anti-murine TCRβ chain antibody were generated by exchanging the constant domain of the hamster immunoglobulin (Ig)G2 for that of the human IgG1.
Understanding antibody-antigen interactions is key for strategic engineering of constructs. Commercially available antibodies against TCR targets can be characterized using proteomics by mass spectrometry. REmAb® de novo protein sequencing provides detailed knowledge of the sequences of antibodies, driving the decisions needed for constructing not only the antibody variants, but also the TCR variants.
Graphical Abstract
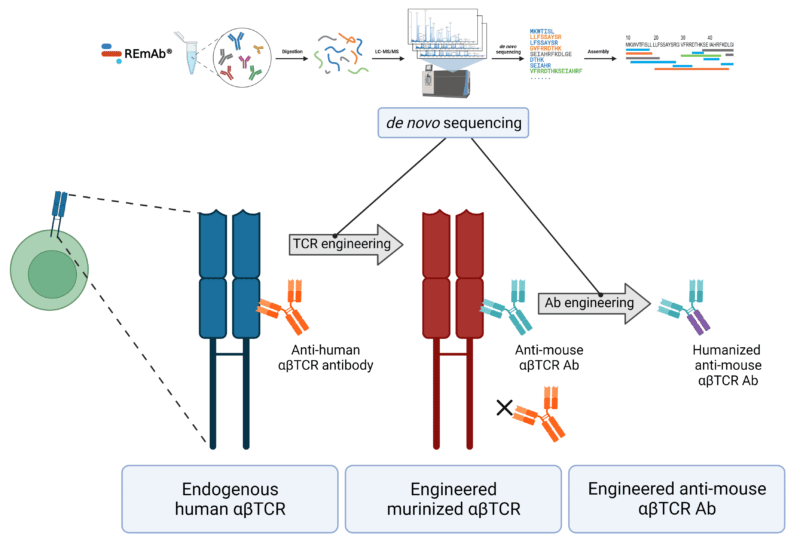
Graphical representation of the directed evolution of the TCR and the humanization of the anti-mouse TCR antibody (Ab). REmAb de novo protein sequencing is incorporated into the engineering pipeline for sequence analysis of antibodies, allowing for mutational analysis of specific amino acids in the TCR and the Ab.
Key Takeaways
- The Kuball Lab characterized and engineered two commercially available antibodies that target TCR-engineered T cells, for applications in TCR-engineered T cell enrichment and depletion.
- Knowledge of the antibody sequences was instrumental in the directed evolution of the TCR and the engineering of the antibody, furthering the characterization of the antibody-antigen binding interface.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

