De novo protein sequencing determined the sequences of all four unique chains of a bispecific antibody, enabling confident advancement to preclinical testing.
Background
UC Davis researcher Dr. Jesse Deere and colleagues focus on T cell recruitment to mediate and respond to chronic viral infections such as HIV and SIV. Their work includes the development of a bispecific T cell engager antibody that has demonstrated efficacy in curing infant animal models with an established SIV infection.
Encouraged by the therapeutic’s success, the researchers were preparing to expand their preclinical studies to evaluate the bispecific antibody’s broader applicability in treating SIV disease.
Challenge
As the researchers prepared to broaden the scope of their preclinical models, they faced a critical obstacle: ensuring they were producing and administering the correct version of the bispecific antibody.
The multi-year and collaborative nature of the project introduced some challenges:
- Optimization efforts generated several sequence iterations due to the need to engineer and refine all four unique antibody chains.
- Documentation inconsistencies left room for uncertainty about the exact sequence of the best-performing bispecific antibody.
Approach
Determining the accurate amino acid sequence was crucial for iterating on this SIV therapeutic and advancing its development. The researchers were confident in their chain pairing, but sought confirmation of the four different chain sequences.
To achieve this, UC Davis researchers turned to Rapid Novor, seeking to derive the sequences of their bispecific antibody using REmAb® de novo monoclonal antibody sequencing service.
Antibody Sequencing Process
De novo sequencing of a bispecific antibody is more complex than sequencing a canonical IgG, as it involves four unique chains instead of two.
Each chain must be sequenced and assembled, using overlapping peptides to ensure the therapeutic’s structure and function are fully understood. Correctly pairing the variable antibody regions with their respective constant regions presents a challenge, particularly due to the high degree of sequence homology and minor sequence differences within the constant regions of this bispecific antibody.
Expert bioinformatic analysis, combined with bottom-up and top-down mass spectrometry methods ensures high sequence coverage and overlapping peptides to accurately assign the correct variable regions to their corresponding constant regions across all unique chains in the bispecific antibody.
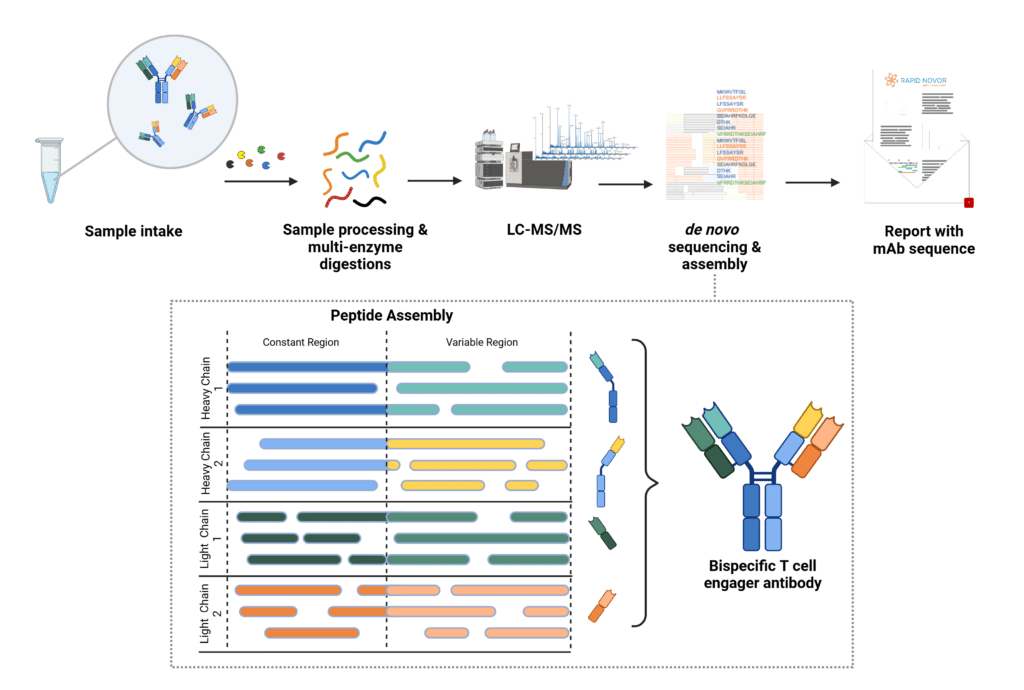
Figure 1. De novo sequencing workflow using the REmAb® platform for monoclonal antibody sequencing, highlighting the de novo sequence assembly and accurate pairing of variable and constant regions for four unique antibody chains in a bispecific T-cell engager.
Outcome
- Using REmAb® monoclonal antibody sequencing, Rapid Novor provided the complete amino acid sequences of all four antibody chains of the bispecific antibody.
- Verifying the sequence prior to production and preclinical testing saved valuable time and resources by ensuring the further development and testing of an antibody having the correct sequence.
Future Directions
With the confirmed sequences of the bispecific antibody in hand, Dr. Jesse Deere’s research team is proceeding with the further development of the bispecific antibody therapeutic and advancing preclinical generalization studies. Their goals include:
Evaluating the application of the bispecific antibody in different age groups, as the SIV disease mechanism may differ between infants and adults.
Assessing the application of the bispecific antibody at different stages of SIV infection, comparing its effectiveness in treating early versus late infections.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

