Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: August 1, 2024
Introduction
Disulfide bonds are among the most critical post-translational modifications (PTMs) for the structural stability and biological function of monoclonal antibodies (mAbs). These bonds form through the oxidation of two thiol groups within cysteine residues, thereby stabilizing mAbs by maintaining the correct domain architecture necessary for antigen binding. Understanding the role of disulfide bonds and the phenomenon of disulfide scrambling, using advanced analytical techniques, is essential for ensuring the stability and functionality of mAbs in research, diagnostic, and therapeutic applications.
Disulfide Bond Structure and Function in Monoclonal Antibodies
Structure
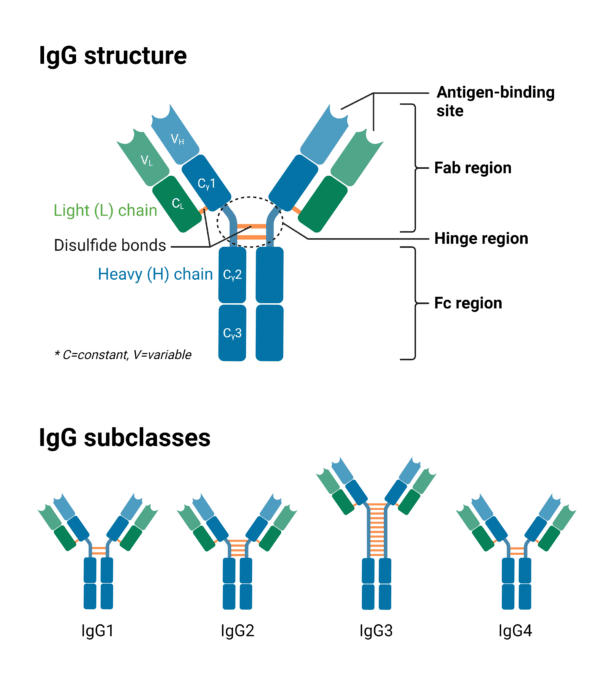
Monoclonal antibodies (mAbs) consist of two identical heavy chains and two identical light chains which make up two distinct regions called the variable region and the constant region (Figure 1). The variable region and the first constant domains of the heavy and light chains make up what is called the Fab (fragment antigen binding) region. The remaining constant domains of the heavy chains make up the Fc (fragment crystallization) region. Between the Fab and Fc region is the hinge region and this is where the heavy chains are linked to each other by two to eleven interchain disulfide bonds, depending on the immunoglobulin G (IgG) subclass. The light chains are also linked to the heavy chains by two interchain disulfide bonds. mAbs also have twelve intrachain disulfide bonds (one per domain) within the heavy and light chain that stabilizes the folded structure of their respective variable and constant domains.
Figure 1: IgG Structure and subclasses highlighting differences in disulfide bonds.
Function
Disulfide bonds play a critical role in the architecture and biological function of mAbs. They maintain the structural integrity and stability of antibodies, contributing significantly to their three-dimensional tertiary and quaternary structures. The correct formation of disulfide bonds is vital for antigen binding and effector functions, including interactions with immune cells and mediation of immune responses. These bonds also enhance the antibody’s resistance to denaturation and protease degradation, thereby increasing its stability in the extracellular environment.
Moreover, disulfide bonds preserve the accurate folding and structural integrity of antibodies, specifically ensuring the stability of the Fab regions and their ability to bind antigens. They facilitate the proper assembly of heavy and light chains into functional antibody molecules, which is essential for the precise recognition and binding of specific antigens. Disulfide bonds in the hinge region allow flexibility in the Fab regions, enabling effective binding to antigens in diverse orientations. However, incorrect configurations of disulfide bonds in antibodies, called disulfide scrambling, can negatively affect the structure, performance, stability, and biological functionality of mAbs.
Disulfide Scrambling in Monoclonal Antibodies
Disulfide scrambling, or disulfide shuffling, refers to the incorrect formation or rearrangement of disulfide bonds within a protein. This can occur during protein synthesis, folding, or storage, leading to the formation of non-native disulfide bonds that can disrupt the protein’s native structure and function. Disulfide bonds formed by disulfide scrambling occur due to free cysteine thiols altering existing disulfide bonds leading to an exchange between thiol groups. Disulfide bond scrambling can occur by three main routes:
- Reactions between free cysteine residues
- Between a free cysteine and a disulfide
- Between two cysteine residues formerly in a disulfide bond
Interchain disulfide bonds are more susceptible to reduction and therefore disulfide scrambling than the intrachain bonds of mAbs. Often disulfide scrambling occurs when mAbs are exposed to denaturation conditions such as:
- Oxidative Stress: When free radicals are present, such as reactive oxygen species (ROS), the cysteine residues can be oxidized which can lead to the formation of non-native disulfide bonds.
- Heat Stress: Exposing mAbs to an increase in temperature can lead to denaturation of the disulfide bonds causing them to reform in mismatched positions that are non-native.
- pH Stress: Similar to heat stress, exposing mAbs to high pH leads to denaturation of the mAb which can cause non-native cysteine pairing.
- Reductive Stress: Exposure to reducing agents can break the native disulfide bonds and lead to the cysteine residues re-pairing in a non-native conformation.
Impact of Disulfide Scrambling on Monoclonal Antibodies
The disulfide scrambling phenomenon can be particularly problematic when producing mAbs for diagnostics, therapeutics, and research since function, stability, efficacy, and safety are of the utmost importance. Disulfide scrambling has been shown to have several negative effects on mAb production:
Decreased Efficacy
Changes in disulfide bond formation can lead to misfolded proteins and, as a result, incorrect tertiary and quaternary mAb structure causing decreased ability to bind target antigen. This leads to a decrease in the overall efficacy of the mAb causing inaccuracies in diagnostics and research and reducing potency in therapeutics.
Decreased Stability
mAbs with changes in disulfide bonds are less stable and more susceptible to degradation by proteases. This leads to a reduction in the shelf life and effectiveness of the mAb when used in diagnostics, research, and therapeutics. Given the high cost of producing mAbs for medicine and research, a decrease in stability will introduce a significant cost burden to these fields. Additionally, a decrease in mAb stability means an increase in dosing for mAb therapeutics leading to a significant burden on patients.
Increased Immunogenicity
Changes in the highly conserved disulfide bonds can lead to mAbs being recognized as a misfolded protein and targeted as foreign by the immune system. This can lead to the production of anti-drug antibodies (ADAs) which neutralize the therapeutic mAbs, reducing their potency and possibly leading to adverse side effects in the patients. For example, studies have shown that disulfide scrambling can lead to increased immunogenicity of mAbs.
Increased Aggregation
Disulfide bond changes in mAbs can also potentially result in aggregation which causes complications in the production process. For example, the interchain disulfide bond in IgG1 antibodies has been shown to significantly reduce aggregation when compared to antibodies that lack this disulfide bond. This can be an expensive problem when considering the cost of producing mAbs for therapeutics, diagnostics, and research. Moreover, this can lead to decreased safety for patients being treated with mAb therapeutics since aggregation can lead to anaphylaxis or serum sickness.
Strategies to Prevent Disulfide Scrambling in Monoclonal Antibodies
Given the adverse effects of disulfide scrambling, implementing comprehensive measures during monoclonal antibody (mAb) production is essential. One effective strategy is genetic engineering. Site-directed mutagenesis during recombinant mAb engineering can replace non-essential cysteine residues with non-cysteine residues, thereby reducing the risk of non-native disulfide bond formation. For example, substituting free cysteine residues that are not involved in native disulfide bonds with serine can prevent unwanted disulfide interactions. Alternatively, introducing additional disulfide bonds at strategic locations in the antibody’s variable or constant regions can enhance structural stability and minimize the risk of scrambling. Either way, sequencing the antibody is vital to making informed cystine engineering decisions to properly manage and optimize disulfide bonds.
Buffer formulation and environmental controls are also critical in preventing disulfide scrambling. The buffer should maintain a controlled redox environment with a balanced ratio of reducing agents (e.g., dithiothreitol [DTT]) and oxidizing agents (e.g., oxidized glutathione) to ensure proper disulfide bond formation. Maintaining a slightly acidic pH (around pH 6.5) is preferred, as alkaline conditions can lead to the deprotonation of free thiols, promoting disulfide exchange and the formation of non-native bonds. Additionally, buffer additives such as glycerol can stabilize the protein structure, antioxidants can protect thiol groups from oxidation, and chelating agents like EDTA can sequester metal ions that might catalyze disulfide bond reshuffling. Maintaining low temperatures and oxygen levels during production is also crucial to prevent scrambling.
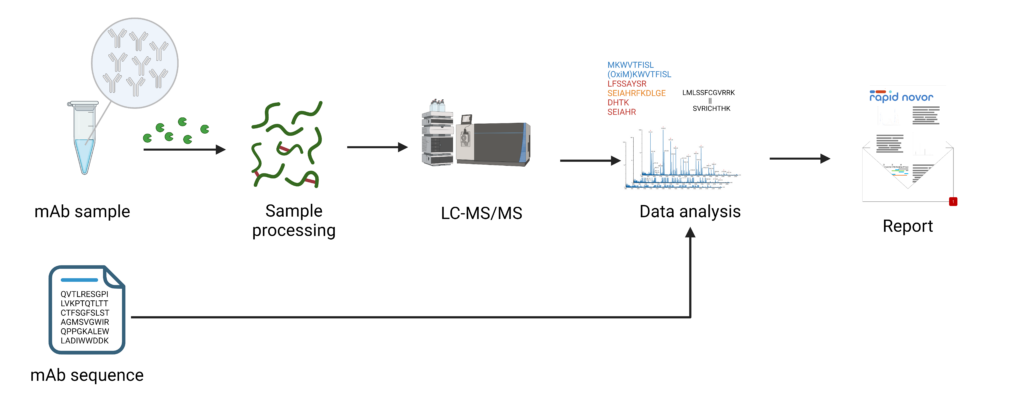
Despite the above precautions, continuous analytical monitoring is vital throughout the mAb production process. Techniques like high-performance liquid chromatography and tandem mass spectrometry (HLC-MS/MS) should be employed to detect and quantify the prevalence of disulfide scrambling (Figure 2). HPLC can separate and quantify different forms of mAbs based on their disulfide bond configurations. MS can provide detailed information about the molecular weight and structure, revealing any misfolded variants. This analytical monitoring enables timely interventions, such as adjusting production conditions or implementing refolding protocols. By using these methods, misfolded antibodies can be refolded into their correct structures, ensuring the production of high-quality, biologically active mAbs. Keep in mind that engineered antibody constructs, such as fragments and nanobodies, require even greater considerations for disulfide bonds than full mAbs.
Figure 2: Disulfide bond analysis workflow using Rapid Novor’s HPLC-MS/MS.
Disulfide Bond Analysis for Monoclonal Antibodies
As mentioned above, ensuring correct disulfide bond formation is crucial for maintaining mAb structural integrity, stability, and therapeutic efficacy. Rapid Novor offers a disulfide bond analysis service which provides precise and accurate insights into the disulfide bond architecture of mAbs using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS).
The service identifies and quantifies both native and non-native disulfide bonds, detecting mispairing that could lead to immunogenicity or reduced potency. Ideal for quality control and regulatory compliance, it delivers reliable disulfide bond analysis to support effective biopharmaceutical development and production.
Discovery scientists who have access to disulfide bond information early in their process can have higher success rates downstream. To characterize the disulfide architecture in your mAb of interest, contact our scientists!
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics