Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: April 1, 2025
Introduction
Disulfide bonds stabilize the tertiary and quaternary structures of proteins by forming covalent linkages between sulfur atoms of cysteine residues. These bonds are vital for the proper folding, stability, and function of many proteins. Engineering disulfide bonds has become an important tool in protein design, as it offers a means of improving the stability, activity, and specificity of proteins used in therapeutic, industrial, and research applications.
Continued advancements in computational tools, experimental techniques, and high throughput methods are making disulfide bond engineering increasingly accessible and effective. Disulfide bond engineering holds great promise in areas such as personalized medicine, protein therapeutics, and the development of novel biomolecules for environmental and industrial applications.
Disulfide Bond Structure and Function
Disulfide bonds are covalent linkages that are responsible for holding proteins in well defined geometric conformations. Upon oxidation, these bonds are formed between the thiol groups of two cysteine residues within a protein or between different protein subunits. These bonds help to stabilize protein structures and are predominantly found in extracellular proteins or proteins that function in harsh conditions.
Disulfide bonds are formed in the endoplasmic reticulum (ER) due to the high presence of oxygen along with chaperones and disulfide isomerases to ensure proper folding and disulfide bond formation (Figure 1). Although a lot of work has been done to predict the effects of altering disulfide bonds, the rules have not been fully characterized and many disulfide bond additions have resulted in decreased stability. Disulfide bond engineering aims to finish characterizing the rules and exploiting them to benefit protein therapeutics.
Figure 1: Disulfide Bond formation in the ER Lumen via protein disulfide isomerase (PDI).
Engineering Disulfide Bonds
Disulfide bond engineering involves the manipulation of cysteine residues to create, eliminate, or modify disulfide bonds within a protein or between protein structures. This process is typically various trial and error experiments to achieve the intended result. Regardless of the intended outcome, disulfide bond engineering can be achieved through various different genetic techniques:
- Site-Directed Mutagenesis: Introducing cysteine residues at specific positions in the protein to form a disulfide bond at a desired location.
- Disulfide Bond Mapping: Mass Spectrometry (MS) can be used to identify and map the disulfide bonds within a protein. Once the positions of disulfide bonds are known, the bonds can be engineered for specific purposes.
- Cysteine Scanning: A systematic approach where one cysteine is introduced at a time into the protein, and the effects on folding and stability are assessed. This technique can be used to identify favorable positions for disulfide bond formation.
- Engineered Disulfide Bond Shuffling: This involves the use of a combinatorial approach to engineer multiple disulfide bonds within a protein. By swapping and shuffling cysteine pairs, you can create a library of proteins with different disulfide bond combinations to identify the most stable or active configuration.
- Protein Engineering Using Computational Methods: Software tools such as Rosetta or FoldX can help design disulfide bonds by predicting the most favorable cysteine pairings that will form a stable structure.
Applications of Disulfide Bond Engineering
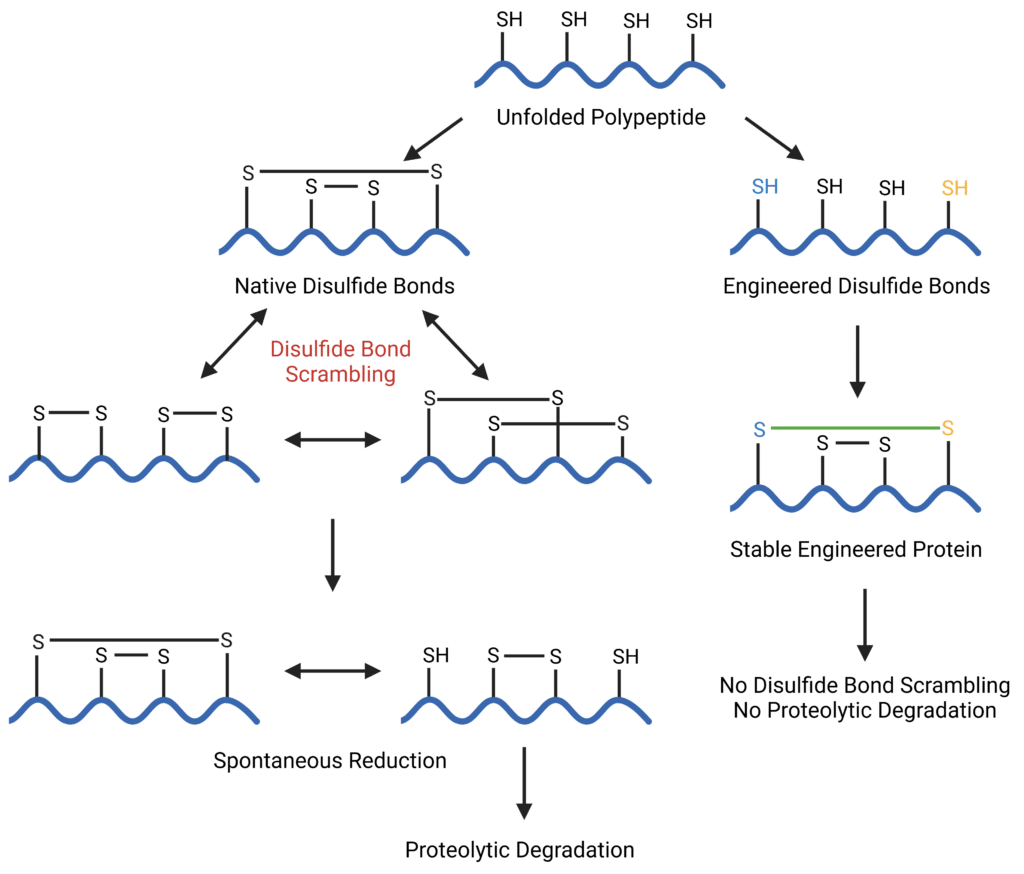
While native disulfide bonds are susceptible to spontaneous reduction and disulfide bond scrambling, making them incredibly prone to proteolytic degradation and misfolding, disulfide bond engineering can be used to eliminate these events (Figure 2). As a result, disulfide bond engineering can elicit a variety of intended effects such as an increase of protein stability, reduction of aggregation, facilitation of protein folding, and alteration of the functions or properties of the protein.
Stability and Solubility Enhancement
Many therapeutic proteins and enzymes are sensitive to environmental conditions such as temperature, pH, or ionic strength. Disulfide bonds can enhance the thermal and chemical stability of these proteins by reducing conformational flexibility and promoting proper folding. For example, scientists demonstrated that a mutated enzyme with an additional disulfide bond has significantly enhanced thermal stability. Additionally, disulfide bonds can also reduce protein aggregation and improve solubility, which is particularly important when producing proteins in bacterial or mammalian expression systems.
Improved Folding
Disulfide bonds are essential for proper protein folding. Engineering disulfide bonds helps stabilize and guide protein folding by forming covalent links between cysteine residues, which lock parts of the protein together. These bonds promote correct folding, reduce misfolding, and enhance stability under environmental stress, such as heat or denaturants. For example, engineering disulfide bonds in recombinant proteins can help in efficient folding of proteins that would otherwise misfold, aiding in the production of functional proteins.
Design of Antibodies and Antibody Fragments
Disulfide bond engineering is widely used to design monoclonal antibodies and antibody fragments. Engineering disulfide bonds between specific regions of antibodies or fragments can enhance their stability and half-life in circulation, as well as improve binding specificity. For example, scientists showed stabilization of adalimumab Fab through the introduction of disulfide bonds between the variable and constant domains. Additionally, single chain variable fragments (scFvs) and nanobodies, derived from camelid antibodies, often require engineered disulfide bonds for stability and function.
Enzyme Engineering
Disulfide bonds can be introduced in enzymes to stabilize their active sites or overall structure, thereby enhancing their catalytic activity or enabling their use in industrial applications where harsh conditions are involved. Enzymes with engineered disulfide bonds are often more robust and can catalyze reactions in environments that are typically not suitable for native enzymes, such as extreme pH or temperature. For example, scientists showed improvement of activity of lytic polysaccharide monooxygenase (LPMO), an enzyme used in the biorefinery industry, through disulfide bond engineering.
Therapeutics Protein Engineering
Disulfide bonds can be engineered into therapeutic proteins (like monoclonal antibodies, enzymes, or cytokines) to enhance their function, stability, half-life, and resistance to degradation in the human body. For example, interleukin-2 (IL-2) is used for cancer immunotherapy, but its instability limits its clinical use. Disulfide bond engineering has been used to stabilize IL-2, improving its stability and pharmacokinetics. Stabilized IL-2 versions result in longer half-lives and enhanced immune activation, making the therapy more effective in cancer treatment.
Vaccine Development
In vaccine development, disulfide bonds can be used to stabilize antigenic proteins, ensuring that they maintain their immunogenic properties even when exposed to the heat, light, or other stress conditions during production, storage, or delivery. For example, with the use of disulfide bond engineering, scientists were able to create a stabilized nanoparticle vaccine for human papillomavirus (HPV). The scientists used structural predicative algorithms such as AlphaFold2 to design disulfide bond locations to stabilize full length HPV antigens displayed on a nanoparticle which enhanced the efficacy of the vaccine.
Figure 2: Schematic representation of native versus engineered disulfide bonds.
Challenges in Disulfide Bond Engineering
The activity of a protein is deeply tied to its 3D structure, and disulfide bonds play a crucial role in maintaining this structure. However, despite their importance, disulfide bonds can be naturally unstable under certain physiological conditions, presenting significant challenges for engineering efforts. As a result, the outcome of disulfide bond engineering is often highly case dependent and requires trial and error experimentation. For example, a series of trial and error experiments done on T4 lysosome, a protein without native disulfide bonds, found that engineered disulfide bonds within the flexible regions of the protein increased stability and those introduced in the rigid regions decreased stability.
When engineering disulfide bonds, several factors must be carefully considered. The strategic placement of cysteine residues is essential to avoid disrupting the protein’s natural conformation and function, as improper placement can lead to misfolding or steric clashes. The redox environment is also crucial, as disulfide bonds require oxidizing conditions for proper formation, often necessitating the use of specific expression systems, such as yeast or mammalian cells. It’s important to consider how disulfide bonds influence folding kinetics, either helping or hindering the protein’s folding process. The engineered bonds should not compromise the protein’s stability, solubility, or activity, and they must enhance stability without causing aggregation.
Finally, it’s not just about altering the protein structure, but also how these changes interact with effector molecules. For example, human A2A adenosine receptor disulfide bonds can modulate ligand binding activity by either changing the conformation of the extracellular loops or disrupting transmembrane domain interactions. Optimizing disulfide bond engineering is a time consuming and resource intensive process, often involving trial and error. However, advances in computational methods, such as molecular dynamics simulations and protein modeling, have made it easier to predict where disulfide bonds might be introduced into a protein without disrupting its function.
Disulfide Bond Analysis with Rapid Novor
Ensuring correct disulfide bond formation is crucial for maintaining protein structural integrity, stability, and therapeutic efficacy.
Rapid Novor offers a disulfide bond analysis service which provides precise and accurate insights into the disulfide bond architecture using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS).
Ideal for quality control and regulatory compliance, it delivers reliable disulfide bond analysis to support effective biopharmaceutical development and production.
Discovery scientists who have access to disulfide bond information early in their process can have higher success rates downstream. To characterize the disulfide architecture in your mAb of interest, contact our scientists!
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics