Ren, J., Liao, X., Lewis, J.M. et al. Generation and optimization of off-the-shelf immunotherapeutics targeting TCR-Vβ2+ T cell malignancy. Nat Commun 15, 519 (2024). https://doi.org/10.1038/s41467-024-44786-2
Key Takeaways
- Targeting a T cell receptor clone TRBV20-1, uniquely expressed by malignant T cells offers an approach to selectively target diseased T cells without affecting normal T cells.
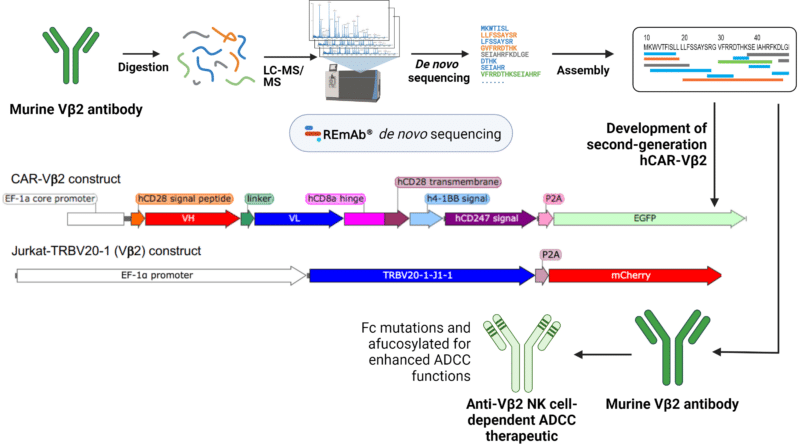
- Through de novo sequencing, a commercially available murine Vβ2 antibody was engineered into a CAR construct, CAR-Vβ2, and a NK-based ADCC agent.
- The humanized CAR-Vβ2 T cells exhibited specific elimination of Vβ2+ malignant T cells, demonstrating efficacy both in vivo and in vitro.
Summary
T cell malignancies pose a complex challenge in developing effective and safe immunotherapies. Despite the success of chimeric antigen receptor (CAR) T-cell therapies, they often lead to the depletion of healthy T cells, increasing susceptibility to infections.
Researchers from the Girardi lab at the Yale School of Medicine aimed to develop novel strategies for the treatment of T cell malignancies without impacting healthy T cells.
Analysis of T cell lymphoma patients revealed that malignant cells express a specific T cell receptor (TCR) clone utilizing a single Vβ-family, known as TRBV20-1. They hypothesized that targeting these dominant TRBVs may be an effective approach to target malignant T cells without impacting normal T cells.
Utilizing REmAb de novo antibody sequencing, the amino acid sequence of a commercially available murine Vβ2 antibody was obtained. This sequence was engineered and incorporated and humanized into a second-generation CAR construct (CAR-Vβ2). CAR-Vβ2 T cells demonstrated specific elimination of Vβ2+ malignant T cells both in vivo and in vitro.
In addition to CAR-T cells, researchers developed a natural killer (NK) cell therapy with antibody-dependent cellular cytotoxicity (ADCC). Using the same commercially available murine Vβ2 antibody, they incorporated Fc-mutations and introduced cell line mutations to engineer afucosylated antibodies with improved ADCC activity.
This innovative method holds promise for advancing targeted therapies against T cell malignancies while minimizing off-target effects on healthy T cells.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

