 Written by:
Written by:
Vanessa Yoon Calvelo, PhD
Published: August 3, 2022
What are Biosimilar Drugs?
Biosimilar drugs, also known as ‘follow-on biologics’ or simply ‘biosimilars’, are biological products shown to be highly similar, but not identical, to their respective reference biologics that are already authorized for sale on the market. Key similarities between biosimilars and their reference products must be demonstrated in terms of quality, safety, and effectiveness, ideally presenting no meaningful clinical differences.
Why are Biosimilars Being Developed?
First-generation protein biologics that were developed using recombinant DNA or hybridoma technologies were essentially copies of human proteins, such as erythropoietin, insulin, growth hormones, and cytokines. These products have transformed treatment regimens of many indications including anaemia, diabetes, cancer, hepatitis, and multiple sclerosis.
However, biologics are generally very expensive due to the high costs in their research and development (R&D), as well as manufacturing. This makes the accessibility of these therapeutics unattainable for many people, even if they are the best course of action for a particular indication. As patents for these first-generation biologics sequentially expire and lose their commercial exclusive rights, biosimilar development opens up in the hopes of increasing the accessibility of equivalent biologics.
Biosimilars are not the Equivalent of Generics
Biosimilars are not ‘generics’. Generics are exact copies of their reference products in terms of their activity and pharmacokinetics. Whereas biosimilars are of biological origin, generics are chemically synthesized making it feasible to reproduce a structurally identical active molecule regardless of differences in manufacturing conditions. This is simply not the case with biosimilars as the active component is often a collection of large protein isoforms rather than a single molecular entity. As a result, the active components between two biosimilars are rarely identical, though they may be highly similar.
Biosimilar Development
Overall, minor differences between the two should not have a relevant impact on the final therapeutic outcome. This is quite different from developing the original reference biologic, which involves establishing the clinical efficacy of the product. As a result, the biosimilar development program operates differently from that of the original reference biologic, as shown in Figure 1 below.
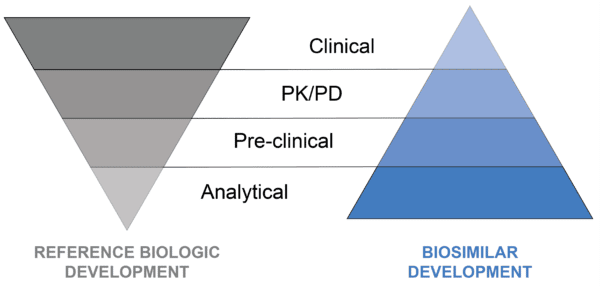
Figure 1. Contrasts between the reference biologic development and biosimilar development programs.
Biosimilar Monoclonal Antibodies
Monoclonal antibodies (mAbs) have become an important class of biologics that are now used in treatment regimens for some indications, such as breast cancer (mAb biologic: Herceptin®, or trastuzumab) and rheumatoid arthritis (mAb biologic: Remicade®, or infliximab). Currently, there are over 100 therapeutic mAbs that are either approved or under regulatory review in the European Union or United States. What once began with murine mAbs has now led to chimeric, humanized, and even mAbs with fully human sequences and reduced immunogenicity due to the steady advancements in R&D. However, the development cost and the attrition rate of novel mAbs are still very high. With the imminent expiration of patents for some mAb products, the idea of developing biosimilar mAbs has drawn attention.
Challenges in Biosimilar mAb Development
In the development of biosimilars, characterization is required to demonstrate that the biosimilar shows high similarity to the original reference biologic. One would think this process would likely be straightforward with licenced mAbs, since they have well-established safety and efficacy profiles for known target indications. However, producing biosimilars of mAbs cannot be equated with the production of small molecules, or even other types of biological molecules, due to their differences in size, complexity, expression capacities, as well as sensitivity to upstream and downstream processing (Figure 2).
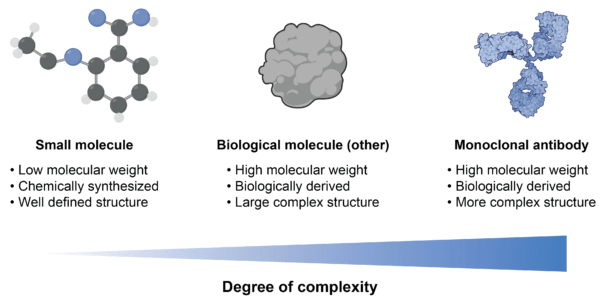
Figure 2. Differences between small molecule, biological molecule, and monoclonal antibody.
As with any protein, mAbs are subject to sequence variations from numerous sources, including differences in the coding DNA, amino acid substitutions during translation, as well as post-translational modifications (PTMs) (Figure 3). The degree of variations and PTMs occurring in mAbs is largely dependent on the type of host cell, culture system and downstream processes and formulations applied. Thus, mAbs and mAb biosimilars are often heterogeneous and include subtle variants that require orthogonal strategies for comparative analysis with the original reference product.
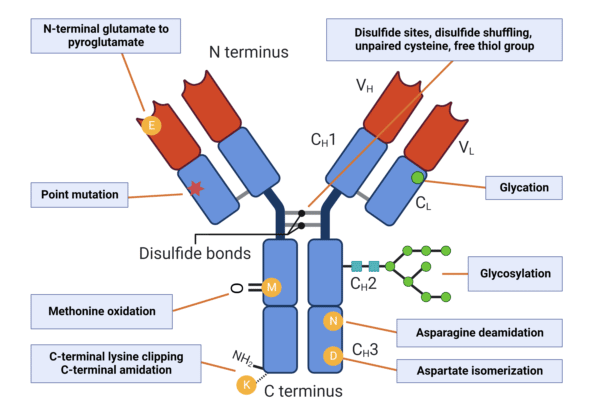
Figure 3. Types of PTMs that increase antibody microheterogeneity.
De Novo Protein Sequencing Solutions in Biosimilar Development
The starting point for biosimilar development is fully characterizing the marketed product. With most reference biologics, however, there is very little access to the proprietary information behind its development. In these cases, our de novo protein sequencing REmAb® technology is the best option to determine the original mAb sequence. When combined with complementary biophysical and biochemical techniques, these methods can streamline the characterization of any mAb biosimilars.
Peptide mapping is widely used in the biopharmaceutical industry during the development of therapeutic proteins, such as mAbs. This technique is often used for product comparability analysis, as it can verify the primary structure of antibody-derived therapeutics, as well as identify sequence variations and monitor PTMs.
Implementing and integrating peptide mapping to a biosimilar R&D program can uncover the true and relevant differences between the biosimilar in question and the original reference product. Our peptide mapping service MATCHmAb® offers a robust approach to analyzing the differences between mAb protein sequences.
Rapid Novor offers a full suite of antibody characterization services using LC-MS to help advance biosimilar drugs from production stages to clinic.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

