 Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: October 18, 2023
Introduction
Protein glycosylation is a ubiquitous post-translational modification that plays a significant role in several cellular and protein functions, including folding, stability, function, and trafficking.
This biochemical process involves the covalent attachment of sugar molecules, known as glycans, to proteins, resulting in the formation of glycoproteins. Glycosylation significantly affects both the structure and function of proteins and influences their biological activity and recognition by other molecules and proteins. In the immune system, antibody glycosylation holds particular significance as it impacts effector functions and antigen-binding capabilities.
Types of Glycans
Glycans are covalently attached to the side chains of specific amino acid residues in proteins through glycosidic bonds. There are two major types of glycosylation: N-linked glycosylation and O-linked glycosylation (Figure 1).
N-Linked Glycans
In N-linked glycosylation, glycans are attached covalently to the nitrogen atom of asparagine (Asn) residues within the Asn-X-Ser/Thr consensus sequence (where X represents any amino acid except proline). The core glycan structure remains consistent across all N-linked glycoproteins, comprising three mannose (Man) residues and two N-acetylglucosamine (GlcNAc) residues, known as Man3GlcNAc2(Asn). N-linked glycans undergo various modifications, leading to the formation of high mannose, complex, and hybrid structures (Figure 1). These modifications introduce significant diversity, contributing to both structural and functional complexities.
O-Linked Glycans
O-linked glycans are covalently linked to the hydroxyl oxygen in the side chain of serine (Ser) and threonine (Thr) amino acid residues. O-linked glycosylation is much more diverse than N-linked glycosylation, due to glycosylation being linked to any monosaccharide, such as mannose, galactose, glucose, fucose, or xylose. However, most O-linked glycans have an N-acetylgalactosamine (GalNAc) core.
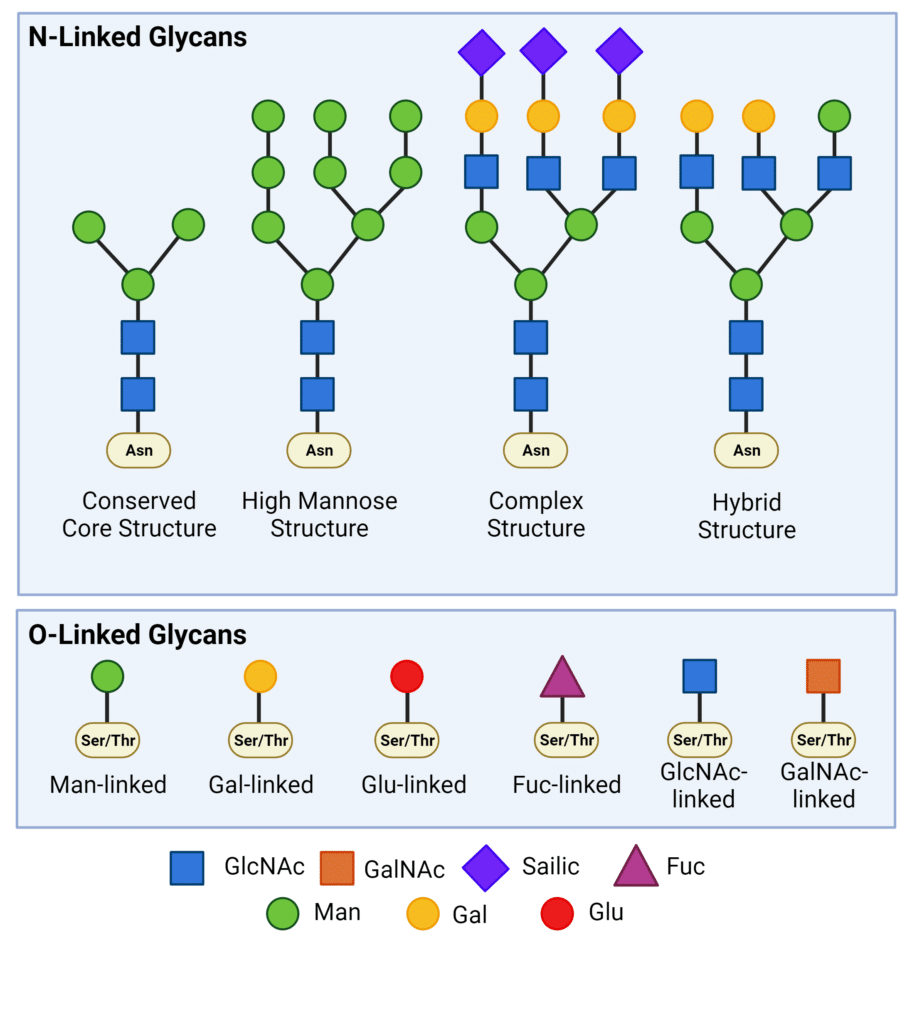
Figure 1. Types of N-linked and O-linked glycans.
How are Proteins Glycosylated?
In eukaryotic cells, such as human and animal cells, protein glycosylation primarily occurs in the secretory pathway, beginning in the endoplasmic reticulum (ER) and completed in the golgi apparatus.
The process begins with the synthesis and translation of a protein in the ER, where the initial stages of protein folding occur. Following translation, glycans are enzymatically attached to amino acid residues, predominantly asparagine (N-linked glycosylation) or serine and threonine residues (O-linked glycosylation). In the Golgi, glycosylated proteins undergo additional modifications, facilitated by various enzymes and protein chaperones which trim or elongate glycans. Although O-linked glycosylation can commence in the ER, it predominantly initiates in the Golgi apparatus after the completion of protein folding.
The compartmentalized nature of protein glycosylation gives rise to a wide range of glycoproteins with diverse structures, leading to unique glycan profiles that reflect distinct protein functions.
Why are Proteins Glycosylated?
The vast diversity of glycans introduces an additional layer of complexity to the proteome, significantly influencing cellular function down to individual protein activity.
At the protein level, glycosylation functions to:
- Promote proper protein folding by enhancing thermal and kinetic stability and reducing the free energy of protein folding.
- Enhances protein stability by stabilizing existing conformations, preventing thermal unfolding, and inhibiting degradation by proteases.
At the cellular level, glycosylation functions in various ways:
- Mediating cell-to-cell recognition in processes such as cell adhesion, immune responses, and cell-to-cell interactions. For example, glycoproteins on the cell surface can serve as receptors for signaling molecules.
- Directing protein trafficking, where proteins destined for secretion of integration into cell membranes undergo specific glycosylation to facilitate their correct trafficking.
Protein Glycosylation in the Immune System
Protein glycosylation plays a critical role in the immune system, affecting various aspects of the immune response, particularly in regards to effector function and antibody binding.
Fc Region Antibody Glycosylation
In the fragment crystalline (Fc) region of antibodies, glycosylation occurs at the highly conserved residue, Asn 297, in all human IgG molecules (Figure 2). Buried between the Fc-domains, glycan modifications can modulate effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and complement activation. Although glycosylation of Asn 297 is conserved, there is significant heterogeneity in IgG glycan modifications. Modifications to the core glycan influences interactions with Fc gamma receptors (FcγR), thereby impacting the recruitment and activity of immune effector cells.
Additionally, Fc N-glycosylation has also been shown to improve antibody stability and half-life, and reduce the aggregation propensity of antibodies.
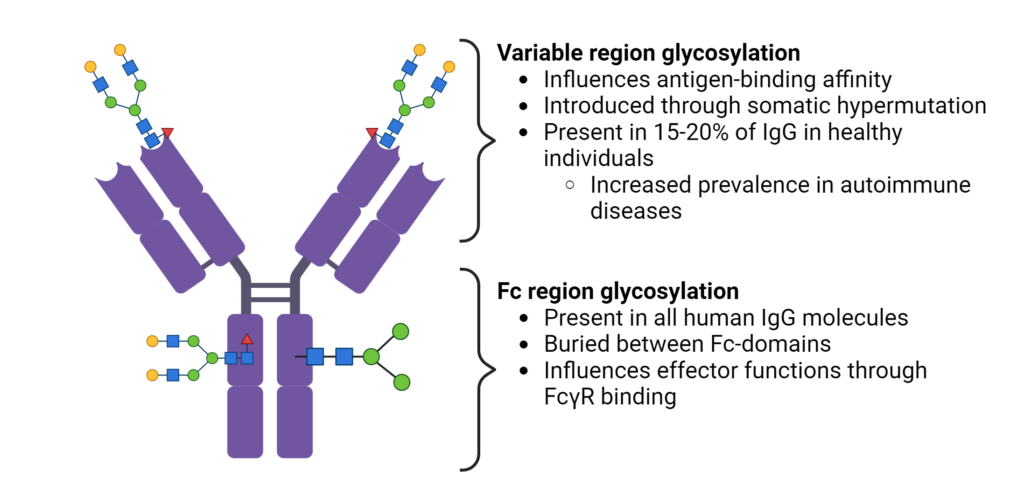
Figure 2. Fc region and variable region glycosylation of IgGs.
Variable Region Antibody Glycosylation
The glycosylation patterns within the variable region of antibodies have a significant impact on their binding affinity to antigens (Figure 2). During antigen-specific immune responses, N-glycosylation sites are introduced into the variable region through somatic hypermutation. The acquired N-linked glycans are selectively chosen during affinity maturation, enhancing the antigen-binding affinity. Additionally, the distinct glycan profiles within different sub-classes of immunoglobulins provides an additional layer of diversification in the antibody repertoire.
It is interesting that variable region glycosylation occurs in 15-20% of IgGs in healthy patients, but is increased in patients with autoimmune disease and other malignancies. Differences in the frequency, and identity of glycan motifs in complementary-determined regions (CDRs) have been observed between healthy and diseased patients (Figure 3). However, the precise role of variable domain glycosylation in disease is incompletely understood. The intricate interplay between glycosylation patterns and immune responses highlights the complexity of antibody functionality in the human body.
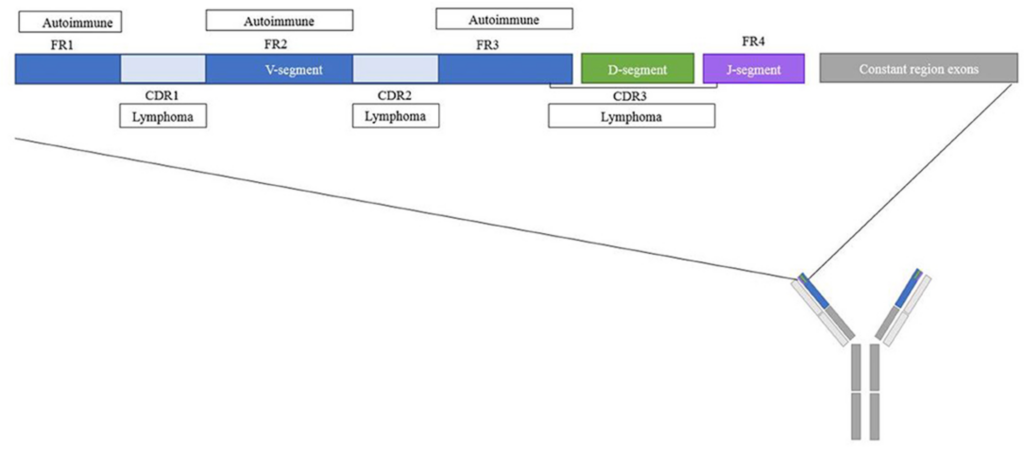
Figure 3. Overview of the variable region of IgGs with frequent glycosylation inpatients with autoimmune diseases and lymphomas (Vletter et al., 2020).
Importance of Understanding Glycan Profiles of Proteins
Given the vast diversity of glycans and their indispensable role in protein and antibody function, it is crucial to understand the distinct glycan profiles for several reasons:
-
- Disease biomarkers: Changes in protein glycosylation patterns, especially in antibodies are associated with various diseases, such as autoimmune diseases. By analyzing glycan profiles, researchers can identify potential biomarkers for disease diagnosis, and identify underlying pathogenic processes.
- Glycoengineering: Understanding glycan profiles allows for the rational modification of glycoproteins, to improve stability, enhance binding affinity, and influence effector functions. Glycoengineering techniques can be used to produce proteins with specific glycan structures tailored for particular functions, or to reduce glycosylation and limit unwanted effects of glycans.
- Recombinant Antibody Production: Monoclonal antibody therapeutics, as well as antibodies for in vitro diagnostics (IVDs) are often produced using recombinant DNA technology in cell lines different from the one the antibody was originally raised in. Switching between different cell lines for recombinant expression, even within the same species, will result in different glycosylation patterns due to differences in glycosylation machinery.
- Quality Control for Biotherapeutics: Glycosylation patterns can vary between batches of biopharmaceuticals, even when the same production process is used. Additionally, regulatory agencies have specific guidelines for glycosylation patterns, and it is essential to ensure compliance with regulatory requirements.
N-Glycan Analysis Service with Rapid Novor
Accelerate antibody development projects by conducting glycan analysis in combination with antibody sequencing and peptide mapping. Discover the locations and relative quantities of common glycans using liquid chromatography and mass spectrometry.
For more information on glycan analysis, contact our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

