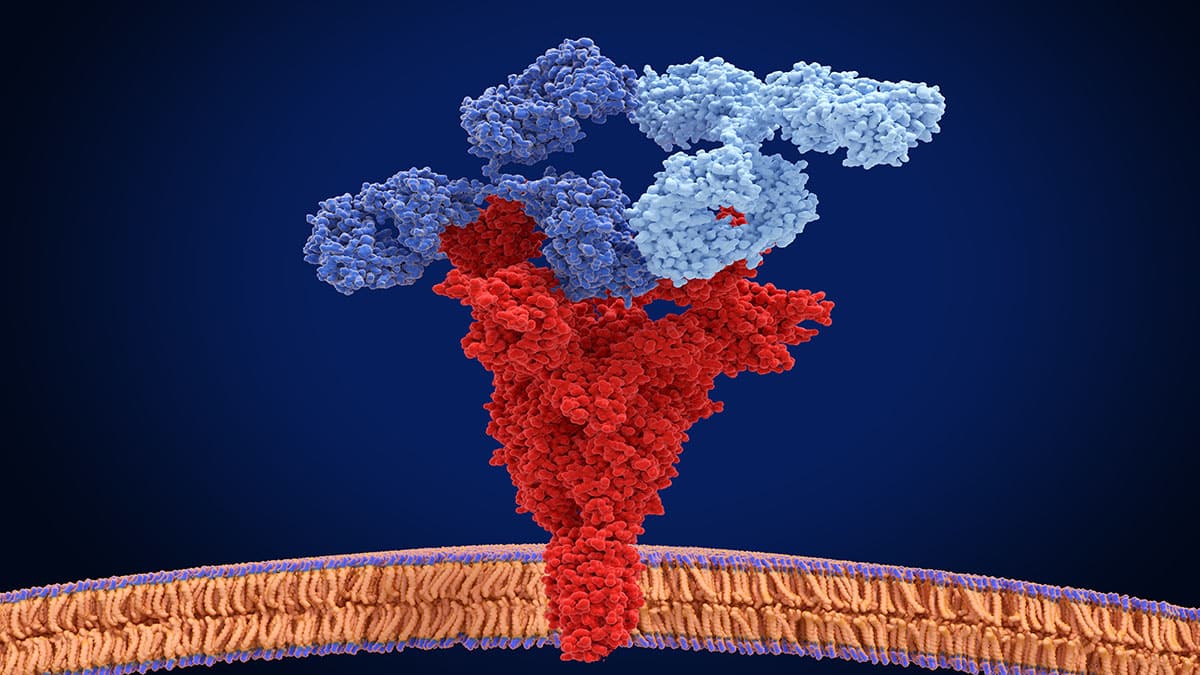
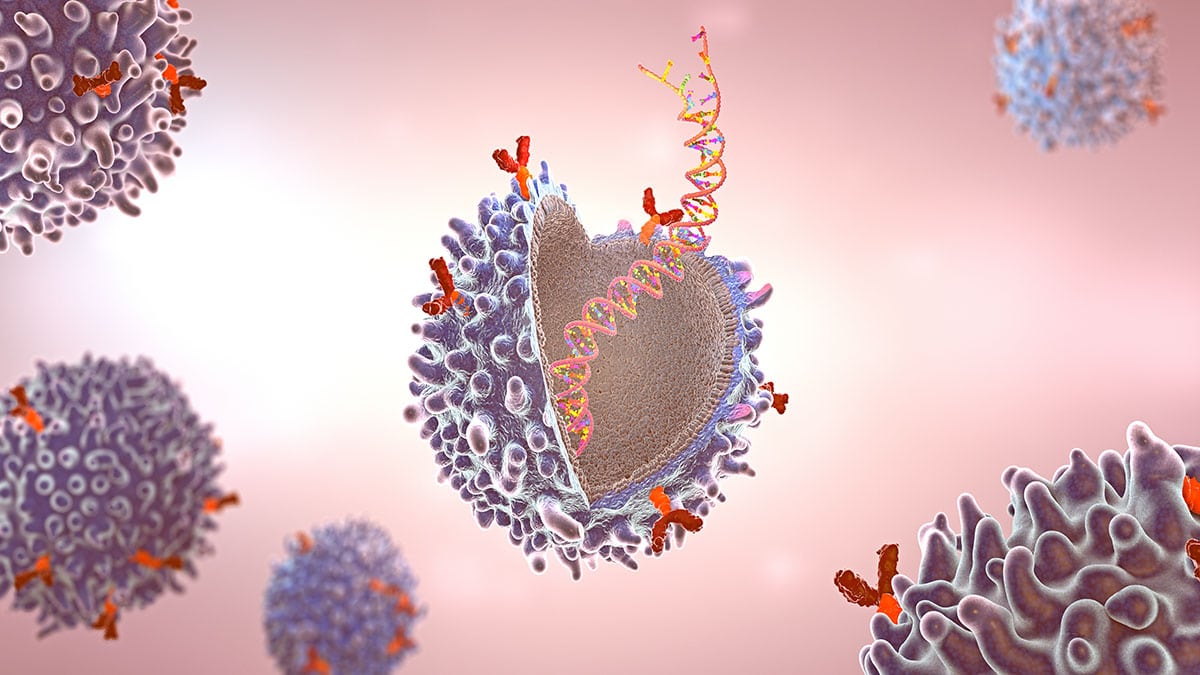
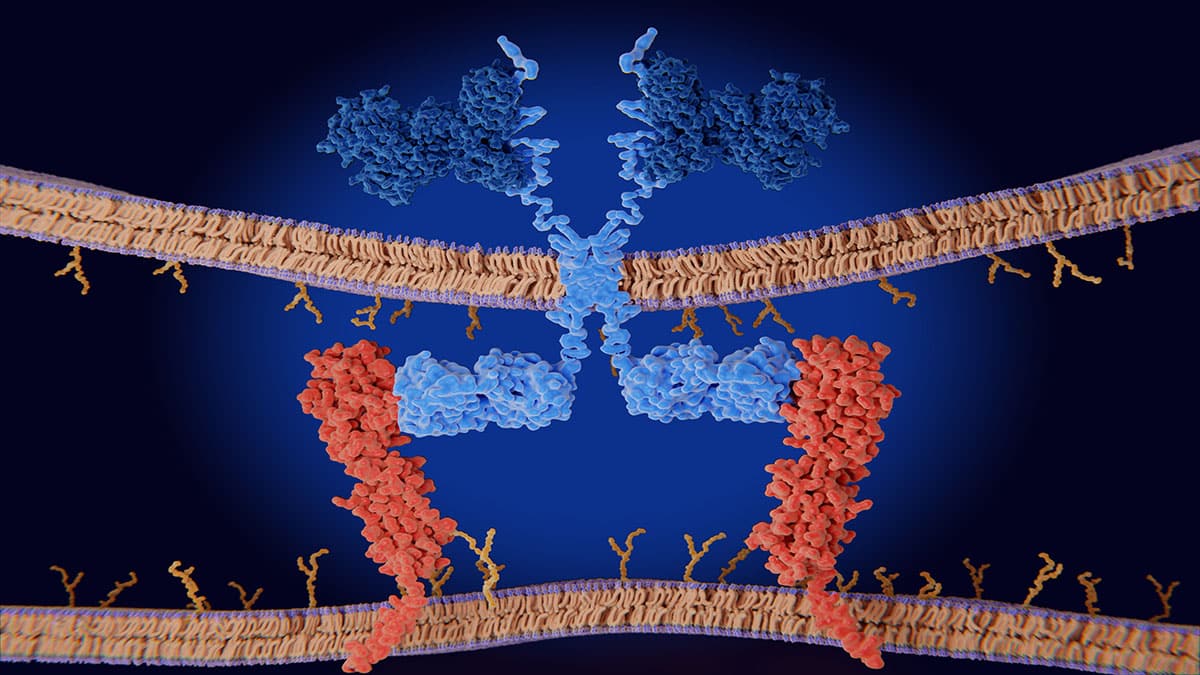
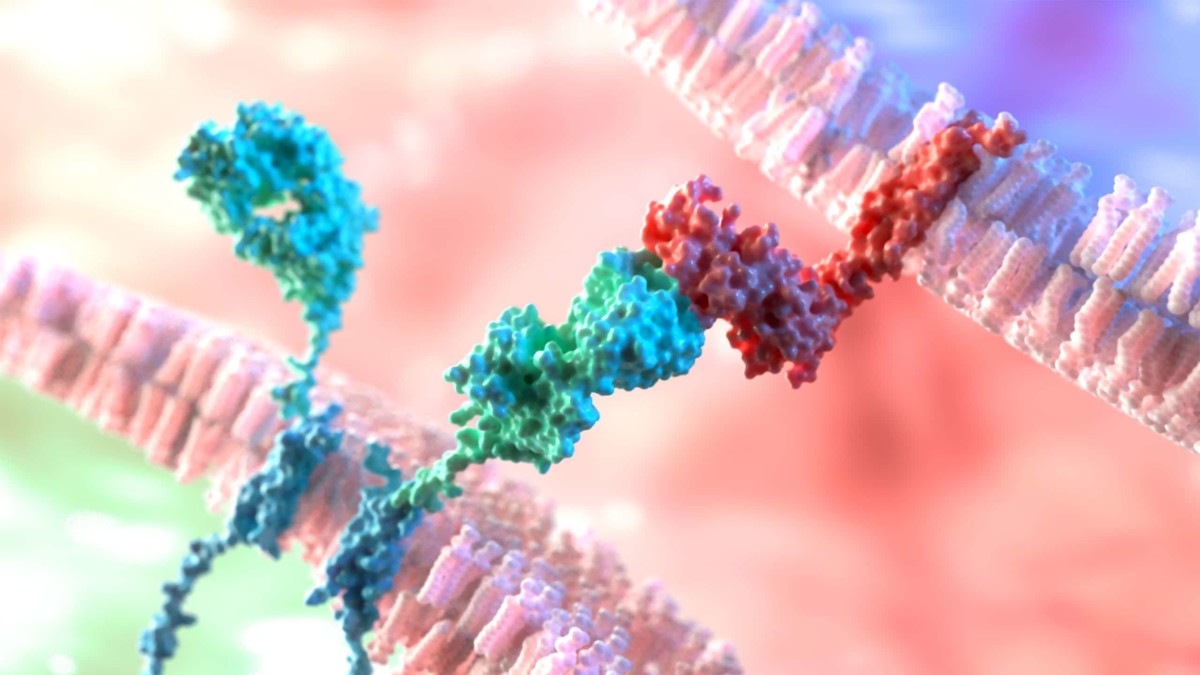
With 22 functional T cell receptor (TCR)Vβ subunit families making up the normal T cell repertoire, signals from these cell surface receptors often determine the fate of normal cells. However, mutations in TCR signaling proteins are frequently associated with peripheral T cell lymphomas (TCLs), including adult T cell leukemia/lymphoma (ATL), which indicates a driving role for TCRs in TCL oncogenesis. As TCL and ATL are clonal in nature, tumour cells typically express a single TCRVβ subunit with no bias in the usage of TCRVβ subunit families. Consequently, targeting the specific TCRVβ subunit presents a promising therapeutic approach that is highly selective and tumour-specific.