Radichev, I. A., Yoon, J., Scott, D. W., Griffin, K. & Savinov, A. Y. Towards Antigen-Specific Tregs for Type 1 Diabetes: Construction and Functional Assessment of Pancreatic Endocrine Marker, HPi2-Based Chimeric Antigen Receptor. Cell Immunol 358, 104224 (2020). https://doi.org/10.1016/j.cellimm.2020.104224.
Abstract
Type 1 diabetes (T1D) is an autoimmune disease that presents itself with insulin deficiency due to the direct elimination of insulin-producing β cells by hyper-activated, autoreactive T effector cells in the pancreatic islets of Langerhans. Currently, insulin is the only approved primary treatment for T1D. Though lifesaving, this form of treatment often requires vigilance, safe handling and administration, and financial investment, placing a tremendous burden on patients and their families.
Addressing the underlying autoimmunity of T1D still remains a critical unmet need. Previous attempts to design immunotherapeutic regimens for clinical utility have been hindered by broad immunosuppressive effects or short-term efficacies. More recent clinical trials have focused on exploiting regulatory T cells (Tregs) as a potential therapy for T1D. A reduction in Treg-dependent suppression of autoimmune responses has been implicated in several autoimmune disorders including T1D. Patients diagnosed with T1D typically present with numerical and functional deficiencies in these regulatory T cells. As a result, Tregs have garnered interest among some research groups, and clinical trials are underway.
The prospect of Treg therapy for T1D is in its early stages. Further optimization is required as the efficacy and specificity of Tregs has yet to meet sufficient levels and safety requirements, respectively. As one option, the Savinov Lab of Sandford Research and the University of South Dakota approached these issues by engineering Tregs with a chimeric antigen receptor (CAR) built to recognize endocrine cells from the islets of Langerhans. The CAR was constructed to contain a single-chain variable fragment (scFv) targeting the human pancreatic endocrine marker HPi2. The variable region sequence of the commercially available anti-HPi2 antibody was first obtained by Rapid Novor’s REmAb® de novo sequencing service, which was subsequently used in the design of the scFv of the HPi2-CAR.
Though the HPi2-CAR-expressing Tregs were non-functional due to the lack of specificity for human pancreatic islets, the study emphasizes the importance of proper selection of CAR recognition drivers for its activity and expandability. De novo protein sequencing can support the design, construction, and validation of CARs, streamlining the process of target identification and elimination. Antibodies with established, specific targets can be sequenced and utilized to engineer the hinge region and antigen-binding domains with antibody fragments and derivatives. With the sequence information in hand, further steps to optimizing a viable therapeutic approach can be more accessible.
Graphical Abstract
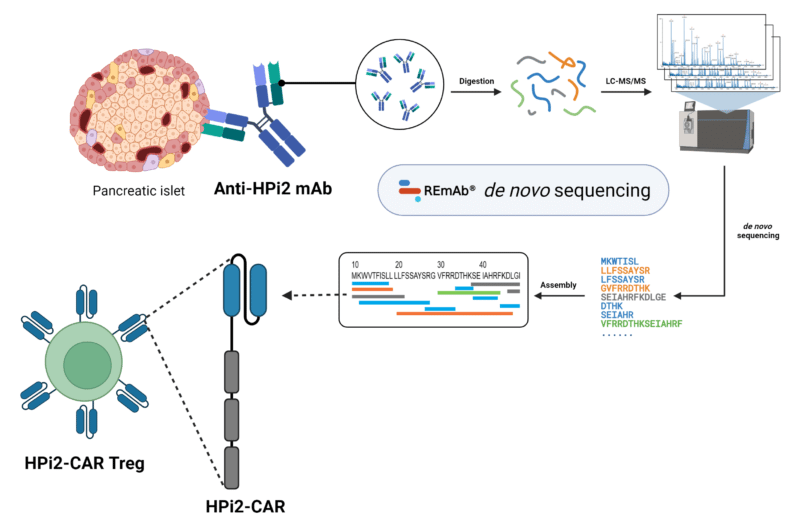
HPi2-CAR engineering workflow involving de novo sequencing of the anti-HPi2 mAb using Rapid Novor’s REmAb® service.
Key Takeaways
- The Savinov Lab obtained the commercially available anti-HPi2 antibody sequence using de novo protein sequencing for the design, construction, and validation of the HPi2-CAR.
- Access to the antibody sequence can provide the essential information for proper selection of recognition drivers and rational engineering of CARs, streamlining the therapeutic research & development pipelines.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

